Proteinase-activated receptors 1 and 4 counter-regulate endostatin and VEGF release from human platelets
- PMID: 15615851
- PMCID: PMC544057
- DOI: 10.1073/pnas.0406682102
Proteinase-activated receptors 1 and 4 counter-regulate endostatin and VEGF release from human platelets
Abstract
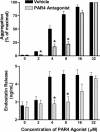
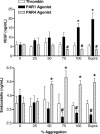
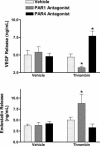
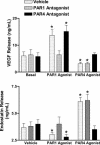
The roles of proteinase-activated receptors (PARs) in platelet functions other than aggregation are not well understood. Among these is the release of factors that regulate the process of angiogenesis, such as endostatin and VEGF, which, respectively, inhibit and promote angiogenesis. PAR1 and PAR4 are expressed on the surface of human platelets and can be activated by thrombin. In the present study, we have attempted to determine the roles of PAR1 and PAR4 in regulating release of endostatin and VEGF from human platelets. Aggregation and endostatin release could be elicited by a specific PAR4 agonist (AYPGKF-NH(2)). The PAR4 agonist concentration dependently suppressed VEGF release. A selective PAR1 agonist (TFLLR-NH(2)) induced platelet aggregation and VEGF release but suppressed endostatin release. Thrombin did not affect endostatin or VEGF release. However, in the presence of a selective PAR1 antagonist (SCH79797), thrombin stimulated endostatin release and suppressed VEGF release. Conversely, in the presence of a selective PAR4 antagonist (transcinnamoyl-YPGKF-NH(2)), thrombin stimulated VEGF release. In vivo, treatment of rats with established gastric ulcers with a PAR1 antagonist each day for 1 wk resulted in a significant retardation of healing. We conclude that PAR1 and PAR4 counter-regulate the release of endostatin and VEGF from platelets. These protease-activated receptors could therefore play a crucial role in regulating angiogenesis and in turn could regulate the processes of wound healing and tumor growth.
Figures

References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

