Microtubule-binding drugs offset tau sequestration by stabilizing microtubules and reversing fast axonal transport deficits in a tauopathy model
- PMID: 15615853
- PMCID: PMC544048
- DOI: 10.1073/pnas.0406361102
Microtubule-binding drugs offset tau sequestration by stabilizing microtubules and reversing fast axonal transport deficits in a tauopathy model
Abstract
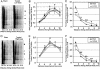
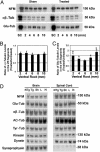
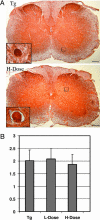
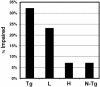
We tested the hypothesis that microtubule (MT)-binding drugs could be therapeutically beneficial in tauopathies by functionally substituting for the MT-binding protein tau, which is sequestered into inclusions of human tauopathies and transgenic mouse models thereof. Transgenic mice were treated for 12 weeks with weekly i.p. injections of 10 or 25 mg/m(2) paclitaxel (Paxceed). Both doses restored fast axonal transport in spinal axons, wherein MT numbers and stable (detyrosinated) tubulins were increased, compared with sham treatment, and only Paxceed ameliorated motor impairments in tau transgenic mice. Thus, MT-stabilizing drugs could have therapeutic potential for treating neurodegenerative tauopathies by offsetting losses of tau function that result from the sequestration of this MT-stabilizing protein into filamentous inclusions.
Figures

References
-
- Lee, V. M.-Y., Goedert, M. & Trojanowski, J. Q. (2001) Annu. Rev. Neurosci. 24, 1121-1159. - PubMed
-
- Morfini, G., Pigino, G., Beffert, U., Busciglio, J. & Brady, S. T. (2002) Neuromol. Med. 2, 89-99. - PubMed
-
- Bramblett, G. T., Goedert, M., Jakes, R., Merrick, S. E., Trojanowski, J. Q. & Lee, V. M.-Y. (1993) Neuron 10, 1089-1099. - PubMed
-
- Lee, V. M.-Y., Balin, B. J., Otvos, L., Jr., & Trojanowski, J. Q. (1991) Science 251, 675-678. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

