Endothelial-specific expression of caveolin-1 impairs microvascular permeability and angiogenesis
- PMID: 15615855
- PMCID: PMC544041
- DOI: 10.1073/pnas.0406092102
Endothelial-specific expression of caveolin-1 impairs microvascular permeability and angiogenesis
Abstract
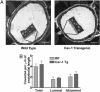
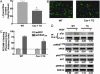
The functions of caveolae and/or caveolins in intact animals are beginning to be explored. Here, by using endothelial cell-specific transgenesis of the caveolin-1 (Cav-1) gene in mice, we show the critical role of Cav-1 in several postnatal vascular paradigms. First, increasing levels of Cav-1 do not increase caveolae number in the endothelium in vivo. Second, despite a lack of quantitative changes in organelle number, endothelial-specific expression of Cav-1 impairs endothelial nitric oxide synthase activation, endothelial barrier function, and angiogenic responses to exogenous VEGF and tissue ischemia. In addition, VEGF-mediated phosphorylation of Akt and its substrate, endothelial nitric oxide synthase, were significantly reduced in VEGF-treated Cav-1 transgenic mice, compared with WT littermates. The inhibitory effect of Cav-1 expression on the Akt-endothelial nitric oxide synthase pathway was specific because VEGF-stimulated phosphorylation of mitogen-activated protein kinase (ERK1/2) was elevated in the Cav-1 transgenics, compared with littermates. These data strongly support the idea that, in vivo, Cav-1 may modulate signaling pathways independent of its essential role in caveolae biogenesis.
Figures



References
-
- Fielding, P. E. & Fielding, C. J. (1995) Biochemistry 34, 14288-14292. - PubMed
-
- Smart, E. J., Ying, Y., Donzell, W. C. & Anderson, R. G. (1996) J. Biol. Chem. 271, 29427-29435. - PubMed
-
- Schnitzer, J. E., Oh, P. & McIntosh, D. P. (1996) Science 274, 239-242. - PubMed
-
- Anderson, R. G., Kamen, B. A., Rothberg, K. G. & Lacey, S. W. (1992) Science 255, 410-411. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- N01 HV 28186/HV/NHLBI NIH HHS/United States
- R01 HL064793/HL/NHLBI NIH HHS/United States
- R01 HL065418/HL/NHLBI NIH HHS/United States
- R01 HL057665/HL/NHLBI NIH HHS/United States
- F32 HL072618/HL/NHLBI NIH HHS/United States
- R01 HL 57665/HL/NHLBI NIH HHS/United States
- R01 HL 64793/HL/NHLBI NIH HHS/United States
- P01 HL 70295/HL/NHLBI NIH HHS/United States
- F32 HL 07216-01/HL/NHLBI NIH HHS/United States
- R01 HL061371/HL/NHLBI NIH HHS/United States
- P01 HL070295/HL/NHLBI NIH HHS/United States
- R01 HL 61371/HL/NHLBI NIH HHS/United States
- N01 HV028186/HL/NHLBI NIH HHS/United States
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Miscellaneous

