Dynamics of GBF1, a Brefeldin A-sensitive Arf1 exchange factor at the Golgi
- PMID: 15616190
- PMCID: PMC551486
- DOI: 10.1091/mbc.e04-07-0599
Dynamics of GBF1, a Brefeldin A-sensitive Arf1 exchange factor at the Golgi
Abstract
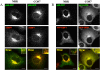
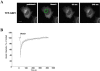
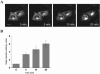
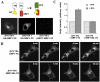
Trafficking through the Golgi apparatus requires members of the Arf family of GTPases, whose activation is regulated by guanine nucleotide exchange factors (GEFs). Once activated, Arf-GTP recruits effectors such as coat complexes and lipid-modifying enzymes to specific membrane sites, creating a domain competent for cargo concentration and transport. GBF1 is a peripherally associated Arf GEF involved in both endoplasmic reticulum-Golgi and intra-Golgi transport. The mechanism of GBF1 binding to membranes is unknown. As a first step to understanding the mechanism of membrane association, we constructed a yellow fluorescent protein-tagged version of GBF1 and performed fluorescence recovery after photobleaching analysis to determine its residence time on Golgi membranes. We find that GBF1 molecules are not stably associated with the Golgi but rather cycle rapidly on and off membranes. The drug brefeldin A (BFA), an uncompetitive inhibitor of the exchange reaction that binds to an Arf-GDP-Arf GEF complex, stabilizes GBF1 on Golgi membranes. Using an in vivo assay to monitor Arf1-GTP levels, we show that GBF1 exchange activity on Arf1 is inhibited by BFA in mammalian cells. These results suggest that an Arf1-GBF1-BFA complex is formed and has a longer residence time on Golgi membranes than GBF1 or Arf1 alone.
Figures




References
-
- Antonny, B., and Schekman, R. (2001). ER export: public transportation by the COPII coach. Curr. Opin. Cell Biol. 13, 438-443. - PubMed
-
- Boman, A. L. (2001). GGA proteins: new players in the sorting game. J. Cell. Sci. 114, 3413-3418. - PubMed
-
- Claude, A., Zhao, B. P., Kuziemsky, C. E., Dahan, S., Berger, S. J., Yan, J. P., Armold, A. D., Sullivan, E. M., and Melancon, P. (1999). GBF 1, A novel Golgi-associated BFA-resistant guanine nucleotide exchange factor that displays specificity for ADP-ribosylation factor 5. J. Cell Biol. 146, 71-84. - PMC - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials

