Novel role for Na,K-ATPase in phosphatidylinositol 3-kinase signaling and suppression of cell motility
- PMID: 15616195
- PMCID: PMC551475
- DOI: 10.1091/mbc.e04-05-0427
Novel role for Na,K-ATPase in phosphatidylinositol 3-kinase signaling and suppression of cell motility
Abstract
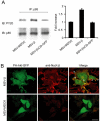
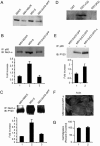
The Na,K-ATPase, consisting of alpha- and beta-subunits, regulates intracellular ion homeostasis. Recent studies have demonstrated that Na,K-ATPase also regulates epithelial cell tight junction structure and functions. Consistent with an important role in the regulation of epithelial cell structure, both Na,K-ATPase enzyme activity and subunit levels are altered in carcinoma. Previously, we have shown that repletion of Na,K-ATPase beta1-subunit (Na,K-beta) in highly motile Moloney sarcoma virus-transformed Madin-Darby canine kidney (MSV-MDCK) cells suppressed their motility. However, until now, the mechanism by which Na,K-beta reduces cell motility remained elusive. Here, we demonstrate that Na,K-beta localizes to lamellipodia and suppresses cell motility by a novel signaling mechanism involving a cross-talk between Na,K-ATPase alpha1-subunit (Na,K-alpha) and Na,K-beta with proteins involved in phosphatidylinositol 3-kinase (PI3-kinase) signaling pathway. We show that Na,K-alpha associates with the regulatory subunit of PI3-kinase and Na,K-beta binds to annexin II. These molecular interactions locally activate PI3-kinase at the lamellipodia and suppress cell motility in MSV-MDCK cells, independent of Na,K-ATPase ion transport activity. Thus, these results demonstrate a new role for Na,K-ATPase in regulating carcinoma cell motility.
Figures






References
-
- Abbott, A., and Ball, W. J., Jr. (1993). The epitope for the inhibitory antibody M7-PB-E9 contains Ser-646 and Asp-652 of the sheep Na+,K(+)-ATPase alpha-subunit. Biochemistry 32, 3511-3518. - PubMed
-
- Anilkumar, G., Rajasekaran, S. A., Wang, S., Hankinson, O., Bander, N. H., and Rajasekaran, A. K. (2003). Prostate-specific membrane antigen association with filamin A modulates its internalization and NAALADase activity. Cancer Res. 63, 2645-2648. - PubMed
-
- Balch, C., and Dedman, J. R. (1997). Annexins II and V inhibit cell migration. Exp. Cell Res. 237, 259-263. - PubMed
-
- Birchmeier, C., Birchmeier, W., and Brand-Saberi, B. (1996). Epithelial-mesenchymal transitions in cancer progression. Acta Anat. 156, 217-226. - PubMed
-
- Blok, L. J., Chang, G. T., Steenbeek-Slotboom, M., van Weerden, W. M., Swarts, H. G., De Pont, J. J., van Steenbrugge, G. J., and Brinkmann, A. O. (1999). Regulation of expression of Na+,K+-ATPase in androgen-dependent and androgen-independent prostate cancer. Br. J. Cancer 81, 28-36. - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

