Identification of novel inhibitors of bacterial translation elongation factors
- PMID: 15616286
- PMCID: PMC538871
- DOI: 10.1128/AAC.49.1.131-136.2005
Identification of novel inhibitors of bacterial translation elongation factors
Abstract
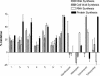
Bacterial elongation factor Tu (EF-Tu) and EF-Ts are interacting proteins involved in polypeptide chain elongation in protein biosynthesis. A novel scintillation proximity assay for the detection of inhibitors of EF-Tu and EF-Ts, as well as the interaction between them, was developed and used in a high-throughput screen of a chemical library. Several compounds from a variety of chemical series with inhibitory properties were identified, including certain indole dipeptides, benzimidazole amidines, 2-arylbenzimidazoles, N-substituted imidazoles, and N-substituted guanidines. The in vitro activities of these compounds were confirmed in a coupled bacterial transcription-translation assay. Several indole dipeptides were identified as inhibitors of bacterial translation, with compound 2 exhibiting a 50% inhibitory concentration of 14 microM and an MIC for S. aureus ATCC 29213 of 5.6 microg/ml. Structure-activity relationship studies around the dipeptidic indoles generated additional analogs with low micromolar MICs for both gram-negative and gram-positive bacteria. To assess the specificity of antibacterial action, these compounds were evaluated in a metabolic labeling assay with Staphylococcus aureus. Inhibition of translation, as well as limited effects on other macromolecular pathways for some of the analogs studied, indicated a possible contribution from a non-target-based antibacterial mechanism of action.
Figures
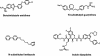
References
-
- Abel, K., M. D. Yoder, R. Hilgenfeld, and F. Jurnak. 1996. An alpha to beta conformational switch in EF-Tu. Structure 4:1153-1159. - PubMed
-
- Berchtold, H., L. Reshetnikova, C. O. A. Reiser, N. K. Schirmer, M. Sprinzl, and R. Hilgenfeld. 1993. Crystal structure of active elongation factor Tu reveals major domain rearrangements. Nature 365:126-132. - PubMed
-
- Heffron, S. E., and F. Jurnak. 2000. Structure of an EF-Tu complex with a thiazolyl peptide antibiotic determined at 2.35 Å resolution: atomic basis for GE2270A inhibition of EF-Tu. Biochemistry 39:37-45. - PubMed
-
- Kariv, I., H. Cao, P. D. Marvil, E. V. Bobkova, Y. E. Bukhtiyarov, Y. P. Yan, U. Patel, L. Coudurier, K. R. Oldenburg, and T. D. Y. Chung. 2001. Identification of the inhibitors of bacterial transcription/translation machinery utilizing the miniaturized 1536-well format screen. J. Biomol. Screen. 6:233-243. - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases

