NF-kappaB activation during Rickettsia rickettsii infection of endothelial cells involves the activation of catalytic IkappaB kinases IKKalpha and IKKbeta and phosphorylation-proteolysis of the inhibitor protein IkappaBalpha
- PMID: 15618150
- PMCID: PMC538953
- DOI: 10.1128/IAI.73.1.155-165.2005
NF-kappaB activation during Rickettsia rickettsii infection of endothelial cells involves the activation of catalytic IkappaB kinases IKKalpha and IKKbeta and phosphorylation-proteolysis of the inhibitor protein IkappaBalpha
Abstract
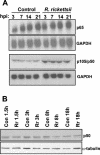
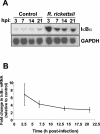
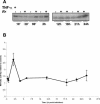
Rocky Mountain spotted fever, a systemic tick-borne illness caused by the obligate intracellular bacterium Rickettsia rickettsii, is associated with widespread infection of the vascular endothelium. R. rickettsii infection induces a biphasic pattern of the nuclear factor-kappaB (NF-kappaB) activation in cultured human endothelial cells (ECs), characterized by an early transient phase at 3 h and a late sustained phase evident at 18 to 24 h. To elucidate the underlying mechanisms, we investigated the expression of NF-kappaB subunits, p65 and p50, and IkappaB proteins, IkappaBalpha and IkappaBbeta. The transcript and protein levels of p50, p65, and IkappaBbeta remained relatively unchanged during the course of infection, but Ser-32 phosphorylation of IkappaBalpha at 3 h was significantly increased over the basal level in uninfected cells concomitant with a significant increase in the expression of IkappaBalpha mRNA. The level of IkappaBalpha mRNA gradually returned toward baseline, whereas that of total IkappaBalpha protein remained lower than the corresponding controls. The activities of IKKalpha and IKKbeta, the catalytic subunits of IkappaB kinase (IKK) complex, as measured by in vitro kinase assays with immunoprecipitates from uninfected and R. rickettsii-infected ECs, revealed significant increases at 2 h after infection. The activation of IKK and early phase of NF-kappaB response were inhibited by heat treatment and completely abolished by formalin fixation of rickettsiae. The IKK inhibitors parthenolide and aspirin blocked the activities of infection-induced IKKalpha and IKKbeta, leading to attenuation of nuclear translocation of NF-kappaB. Also, increased activity of IKKalpha was evident later during the infection, coinciding with the late phase of NF-kappaB activation. Thus, activation of catalytic components of the IKK complex represents an important upstream signaling event in the pathway for R. rickettsii-induced NF-kappaB activation. Since NF-kappaB is a critical regulator of inflammatory genes and prevents host cell death during infection via antiapoptotic functions, selective inhibition of IKK may provide a potential target for enhanced clearance of rickettsiae and an effective strategy to reduce inflammatory damage to the host during rickettsial infections.
Figures




Similar articles
-
Proteasome-independent activation of nuclear factor kappaB in cytoplasmic extracts from human endothelial cells by Rickettsia rickettsii.Infect Immun. 1998 May;66(5):1827-33. doi: 10.1128/IAI.66.5.1827-1833.1998. Infect Immun. 1998. PMID: 9573057 Free PMC article.
-
Regulation and function of IKK and IKK-related kinases.Sci STKE. 2006 Oct 17;2006(357):re13. doi: 10.1126/stke.3572006re13. Sci STKE. 2006. PMID: 17047224 Review.
-
Expression and secretion of chemotactic cytokines IL-8 and MCP-1 by human endothelial cells after Rickettsia rickettsii infection: regulation by nuclear transcription factor NF-kappaB.Int J Med Microbiol. 2005 Aug;295(4):267-78. doi: 10.1016/j.ijmm.2005.05.006. Int J Med Microbiol. 2005. PMID: 16128401
-
Inhibition of IKK down-regulates antigen + IgE-induced TNF production by mast cells: a role for the IKK-IkappaB-NF-kappaB pathway in IgE-dependent mast cell activation.J Leukoc Biol. 2005 Jun;77(6):975-83. doi: 10.1189/jlb.0204115. Epub 2005 Mar 22. J Leukoc Biol. 2005. PMID: 15784689
-
Phosphorylation meets ubiquitination: the control of NF-[kappa]B activity.Annu Rev Immunol. 2000;18:621-63. doi: 10.1146/annurev.immunol.18.1.621. Annu Rev Immunol. 2000. PMID: 10837071 Review.
Cited by
-
Infection of human endothelial cells with spotted Fever group rickettsiae stimulates cyclooxygenase 2 expression and release of vasoactive prostaglandins.Infect Immun. 2006 Sep;74(9):5067-74. doi: 10.1128/IAI.00182-06. Infect Immun. 2006. PMID: 16926398 Free PMC article.
-
Pathogenesis of Rickettsial Diseases: Pathogenic and Immune Mechanisms of an Endotheliotropic Infection.Annu Rev Pathol. 2019 Jan 24;14:127-152. doi: 10.1146/annurev-pathmechdis-012418-012800. Epub 2018 Aug 27. Annu Rev Pathol. 2019. PMID: 30148688 Free PMC article. Review.
-
Molecular mechanisms of host-pathogen interactions and their potential for the discovery of new drug targets.Curr Drug Targets. 2008 Feb;9(2):150-7. doi: 10.2174/138945008783502449. Curr Drug Targets. 2008. PMID: 18288966 Free PMC article. Review.
-
Salmonella Typhi serine threonine kinase T4519 induces lysosomal membrane permeabilization by manipulating Toll-like receptor 2-Cystatin B-Cathepsin B-NF-κB-reactive oxygen species pathway and promotes survival within human macrophages.PLoS Pathog. 2025 Apr 1;21(4):e1013041. doi: 10.1371/journal.ppat.1013041. eCollection 2025 Apr. PLoS Pathog. 2025. PMID: 40168426 Free PMC article.
-
Differential utilization of nuclear factor-kappaB signaling pathways for gingival epithelial cell responses to oral commensal and pathogenic bacteria.Oral Microbiol Immunol. 2008 Apr;23(2):119-26. doi: 10.1111/j.1399-302X.2007.00398.x. Oral Microbiol Immunol. 2008. PMID: 18279179 Free PMC article.
References
-
- Bierhaus, A., and P. P. Naworth. 2003. Modulation of the vascular endothelium during infection: the role of NF-κB activation. Contrib. Microbiol. 10:86-105. - PubMed
-
- Denk, A., M. Goebeler, S. Schmid, I. Berberich, O. Ritz, D. Lindemann, S. Ludwig, and T. Wirth. 2001. Activation of NF-κB via the IκB kinase complex is both essential and sufficient for proinflammatory gene expression in primary endothelial cells. J. Biol. Chem. 276:28451-28458. - PubMed
-
- DiDonato, J. A. 2000. Assaying for IκB kinase activity. Methods Enzymol. 322:393-400. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

