Chemokine expression in the monocytic cell line THP-1 in response to purified shiga toxin 1 and/or lipopolysaccharides
- PMID: 15618178
- PMCID: PMC538957
- DOI: 10.1128/IAI.73.1.403-412.2005
Chemokine expression in the monocytic cell line THP-1 in response to purified shiga toxin 1 and/or lipopolysaccharides
Abstract
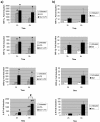
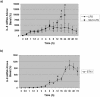
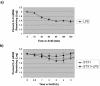
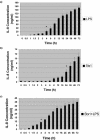
Infections with Shiga toxin (Stx)-producing bacteria are associated with bloody diarrhea and postdiarrheal sequelae, including hemolytic uremic syndrome and central nervous system (CNS) abnormalities. Stx-induced intestinal, renal, and CNS vascular lesions may involve a localized production of proinflammatory cytokines in target organs, as tumor necrosis factor-alpha (TNF-alpha) and interleukin-1beta (IL-1beta) up-regulate Stx receptor globotriaosylceramide (Gb(3)) expression on vascular endothelial cells. However, leukocyte recruitment to injured sites may also exacerbate vascular damage. A cytokine macroarray analysis of transcripts derived from macrophage-like THP-1 cells treated with Stx1, lipopolysaccharides (LPS), or both demonstrated a consistent up-regulation of TNF-alpha, IL-1beta, and four genes encoding the chemokines interleukin-8 (IL-8), macrophage inflammatory protein-1alpha (MIP-1alpha), MIP-1beta, and growth-related oncogene beta (GRO-beta). Real-time PCR analysis verified the macroarray results. Northern blot analyses after the addition of the transcriptional inhibitor actinomycin D revealed increased IL-8 mRNA stability in THP-1 cells treated with Stx1 or Stx1 plus LPS. Finally, enzyme-linked immunosorbent assay data for Stx1- plus LPS-treated cells demonstrated a poor correlation between IL-8, MIP-1alpha, MIP-1beta, and GRO-beta mRNA levels and protein production, indicating a posttranscriptional regulatory effect. Our data suggest that in response to Stx1 and LPS, macrophages may be a source of chemokines that promote tissue damage through leukocyte recruitment and activation.
Figures

Similar articles
-
Regulation of proinflammatory cytokine expression by Shiga toxin 1 and/or lipopolysaccharides in the human monocytic cell line THP-1.Infect Immun. 2004 May;72(5):2618-27. doi: 10.1128/IAI.72.5.2618-2627.2004. Infect Immun. 2004. PMID: 15102770 Free PMC article.
-
Dobutamine modulates lipopolysaccharide-induced macrophage inflammatory protein-1alpha and interleukin-8 production in human monocytes.Anesth Analg. 2003 Jul;97(1):210-5, table of contents. doi: 10.1213/01.ane.0000066257.38180.04. Anesth Analg. 2003. PMID: 12818968
-
The Effects of Shiga Toxin 1, 2 and Their Subunits on Cytokine and Chemokine Expression by Human Macrophage-Like THP-1 Cells.Toxins (Basel). 2015 Oct 9;7(10):4054-66. doi: 10.3390/toxins7104054. Toxins (Basel). 2015. PMID: 26473922 Free PMC article.
-
Comparative evaluation of apoptosis induced by Shiga toxin 1 and/or lipopolysaccharides in human monocytic and macrophage-like cells.Microb Pathog. 2005 Feb-Mar;38(2-3):63-76. doi: 10.1016/j.micpath.2004.12.003. Microb Pathog. 2005. PMID: 15748808
-
Regulation of cytokine and chemokine expression by the ribotoxic stress response elicited by Shiga toxin type 1 in human macrophage-like THP-1 cells.Infect Immun. 2012 Jun;80(6):2109-20. doi: 10.1128/IAI.06025-11. Epub 2012 Mar 19. Infect Immun. 2012. PMID: 22431646 Free PMC article.
Cited by
-
Transcriptional profiling of the LPS induced NF-kappaB response in macrophages.BMC Immunol. 2007 Jan 12;8:1. doi: 10.1186/1471-2172-8-1. BMC Immunol. 2007. PMID: 17222336 Free PMC article.
-
Shiga toxin 1-induced proinflammatory cytokine production is regulated by the phosphatidylinositol 3-kinase/Akt/mammalian target of rapamycin signaling pathway.Infect Immun. 2009 Sep;77(9):3919-31. doi: 10.1128/IAI.00738-09. Epub 2009 Jul 13. Infect Immun. 2009. PMID: 19596774 Free PMC article.
-
Shiga toxins expressed by human pathogenic bacteria induce immune responses in host cells.J Microbiol. 2013 Dec;51(6):724-30. doi: 10.1007/s12275-013-3429-6. Epub 2013 Dec 19. J Microbiol. 2013. PMID: 24385347 Review.
-
Ubiquitin-Specific Protease 14 Negatively Regulates Toll-Like Receptor 4-Mediated Signaling and Autophagy Induction by Inhibiting Ubiquitination of TAK1-Binding Protein 2 and Beclin 1.Front Immunol. 2017 Dec 15;8:1827. doi: 10.3389/fimmu.2017.01827. eCollection 2017. Front Immunol. 2017. PMID: 29326710 Free PMC article.
-
Shiga toxin remodels the intestinal epithelial transcriptional response to Enterohemorrhagic Escherichia coli.PLoS Pathog. 2021 Feb 2;17(2):e1009290. doi: 10.1371/journal.ppat.1009290. eCollection 2021 Feb. PLoS Pathog. 2021. PMID: 33529199 Free PMC article.
References
-
- Auwerx, J. 1991. The human leukemia cell line, THP-1: a multifaceted model for the study of monocyte-macrophage differentiation. Experientia 44:22-31. - PubMed
-
- Cockwell, P., A. J. Howie, D. Adu, and C. O. Savage. 1998. In situ analysis of C-C chemokine mRNA in human glomerulonephritis. Kidney Int. 54:827-836. - PubMed
-
- Endo, Y., K. Tsurugi, T. Yutsudo, Y. Takeda, T. Ogasawara, and K. Igarashi. 1988. Site of action of a Vero toxin (VT2) from Escherichia coli O157:H7 and Shiga toxin on eukaryotic ribosomes. RNA N-glycosidase activity of the toxins. Eur. J. Biochem. 171:45-50. - PubMed
-
- Fitzpatrick, M. M., V. Shah, R. S. Trompeter, M. J. Dillon, and T. M. Barratt. 1992. Interleukin-8 and polymorphonuclear leucocyte activation in hemolytic uremic syndrome of childhood. Kidney Int. 42:951-956. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

