Mucosal FOXP3-expressing CD4+ CD25high regulatory T cells in Helicobacter pylori-infected patients
- PMID: 15618192
- PMCID: PMC538965
- DOI: 10.1128/IAI.73.1.523-531.2005
Mucosal FOXP3-expressing CD4+ CD25high regulatory T cells in Helicobacter pylori-infected patients
Abstract
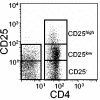
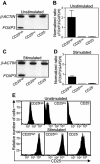
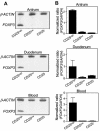
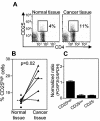
Helicobacter pylori chronically colonizes the stomach and duodenum and causes peptic ulcers or gastric adenocarcinoma in 10 to 20% of infected individuals. We hypothesize that the inability of patients to clear H. pylori infections is a consequence of active suppression of the immune response. Here we show that H. pylori-infected individuals have increased frequencies of CD4(+) CD25(high) T cells in both the stomach and duodenal mucosa compared to uninfected controls. These cells have the phenotype of regulatory T cells, as they express FOXP3, a key gene for the development and function of regulatory T cells, as well as high levels of the cytotoxic T lymphocyte-associated antigen 4 (CTLA-4) protein. In contrast, mucosal CD4(+) CD25(low) and CD4(+) CD25(-) cells express little FOXP3 mRNA and low levels of the CTLA-4 protein. Mucosal CD4(+) CD25(high) T cells are present in individuals with asymptomatic H. pylori infections as well as in duodenal ulcer patients. The frequencies of CD4(+) CD25(high) cells are also increased in the stomachs of H. pylori-infected patients with gastric adenocarcinoma, particularly in cancer-affected tissues. These findings suggest that regulatory T cells may suppress mucosal immune responses and thereby contribute to the persistence of H. pylori infections.
Figures

References
-
- Baecher-Allan, C., J. A. Brown, G. J. Freeman, and D. A. Hafler. 2001. CD4+CD25high regulatory cells in human peripheral blood. J. Immunol. 167:1245-1253. - PubMed
-
- Belkaid, Y., C. A. Piccirillo, S. Mendez, E. M. Shevach, and D. L. Sacks. 2002. CD4+CD25+ regulatory T cells control Leishmania major persistence and immunity. Nature 420:502-507. - PubMed
-
- Cao, D., V. Malmstrom, C. Baecher-Allan, D. Hafler, L. Klareskog, and C. Trollmo. 2003. Isolation and functional characterization of regulatory CD25bright CD4+ T cells from the target organ of patients with rheumatoid arthritis. Eur. J. Immunol. 33:215-223. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials

