Replication dynamics of Mycobacterium tuberculosis in chronically infected mice
- PMID: 15618194
- PMCID: PMC538940
- DOI: 10.1128/IAI.73.1.546-551.2005
Replication dynamics of Mycobacterium tuberculosis in chronically infected mice
Abstract
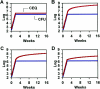
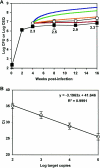
The dynamics of host-pathogen interactions have important implications for the design of new antimicrobial agents to treat chronic infections such as tuberculosis (TB), which is notoriously refractory to conventional drug therapy. In the mouse model of TB, an acute phase of exponential bacterial growth in the lungs is followed by a chronic phase characterized by relatively stable numbers of bacteria. This equilibrium could be static, with little ongoing replication, or dynamic, with continuous bacterial multiplication balanced by bacterial killing. A static model predicts a close correspondence between "viable counts" (live bacteria) and "total counts" (live plus dead bacteria) in the lungs over time. A dynamic model predicts the divergence of total counts and viable counts over time due to the accumulation of dead bacteria. Here, viable counts are defined as bacterial CFU enumerated by plating lung homogenates; total counts are defined as bacterial chromosome equivalents (CEQ) enumerated by using quantitative real-time PCR. We show that the viable and total bacterial counts in the lungs of chronically infected mice do not diverge over time. Rapid degradation of dead bacteria is unlikely to account for the stability of bacterial CEQ numbers in the lungs over time, because treatment of mice with isoniazid for 8 weeks led to a marked reduction in the number of CFU without reducing the number of CEQ. These observations support the hypothesis that the stable number of bacterial CFU in the lungs during chronic infection represents a static equilibrium between host and pathogen.
Figures


References
-
- Cole, S. T. 2002. Comparative and functional genomics of the Mycobacterium tuberculosis complex. Microbiology 148:2919-2928. - PubMed
-
- de Wit, D., M. Wootton, J. Dhillon, and D. A. Mitchison. 1995. The bacterial DNA content of mouse organs in the Cornell model of dormant tuberculosis. Tuber. Lung Dis. 76:555-562. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

