Cytolethal distending toxin from Shiga toxin-producing Escherichia coli O157 causes irreversible G2/M arrest, inhibition of proliferation, and death of human endothelial cells
- PMID: 15618195
- PMCID: PMC538959
- DOI: 10.1128/IAI.73.1.552-562.2005
Cytolethal distending toxin from Shiga toxin-producing Escherichia coli O157 causes irreversible G2/M arrest, inhibition of proliferation, and death of human endothelial cells
Abstract
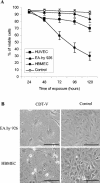
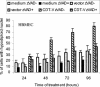
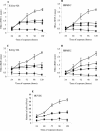
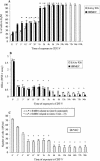
Recently, cytolethal distending toxin V (CDT-V), a new member of the CDT family, was identified in Shiga toxin-producing Escherichia coli (STEC) O157 and particular non-O157 serotypes. Here we investigated the biological effects of CDT-V from STEC O157:H(-) (strain 493/89) on human endothelial cells, which are believed to be major pathogenetic targets in severe STEC-mediated diseases. CDT-V caused dose-dependent G(2)/M cell cycle arrest leading to distension, inhibition of proliferation, and death in primary human umbilical vein endothelial cells (HUVEC) and two endothelial cell lines, EA.hy 926 cells (HUVEC derived) and human brain microvascular endothelial cells (HBMEC). The cell cycle effects of CDT-V were cell type specific. In HUVEC and EA.hy 926 cells, CDT-V caused a slowly developing but persistent G(2)/M block which resulted in delayed nonapoptotic cell death. In contrast, in HBMEC, CDT-V induced a rapidly evolving but transient G(2)/M block which was followed by progressive, mostly apoptotic cell death. In both HBMEC and EA.hy 926 cells, G(2)/M arrest was preceded by the early accumulation of a phosphorylated inactive form of cdc2 kinase. Significant G(2)/M arrest and inhibition of proliferation in both HUVEC and each of the endothelial cell lines were induced by 2 to 15 min of exposure to CDT-V, indicating that the effects of the toxin are irreversible. CDT-V-treated HBMEC and EA.hy 926 cells displayed fragmented nuclei and expressed phosphorylated histone protein H2AX, indicative of DNA damage followed by a DNA repair response. Our data demonstrate that CDT-V causes irreversible damage to human endothelial cells and thus may contribute to the pathogenesis of STEC-mediated diseases.
Figures



References
-
- Bielaszewska, M., and H. Karch. 2000. Non-O157:H7 Shiga toxin (verocytotoxin)-producing Escherichia coli strains: epidemiological significance and microbiological diagnosis. World J. Microbiol. Biotechnol. 16:711-718.
-
- Bitzan, M., and D. M. W. M. Te Loo. 2003. Interaction of Shiga toxin with endothelial cells. Methods Mol. Med. 73:243-262. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Miscellaneous

