Microelectrode array recordings of cultured hippocampal networks reveal a simple model for transcription and protein synthesis-dependent plasticity
- PMID: 15618268
- PMCID: PMC1456059
- DOI: 10.1113/jphysiol.2004.077446
Microelectrode array recordings of cultured hippocampal networks reveal a simple model for transcription and protein synthesis-dependent plasticity
Abstract
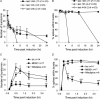
A simplified cell culture system was developed to study neuronal plasticity. As changes in synaptic strength may alter network activity patterns, we grew hippocampal neurones on a microelectrode array (MEA) and monitored their collective behaviour with 60 electrodes simultaneously. We found that exposure of the network for 15 min to the GABA(A) receptor antagonist bicuculline induced an increase in synaptic efficacy at excitatory synapses that was associated with an increase in the frequency of miniature AMPA receptor-mediated EPSCs and a change in network activity from uncoordinated firing of neurones (lacking any recognizable pattern) to a highly organized, periodic and synchronous burst pattern. Induction of recurrent synchronous bursting was dependent on NMDA receptor activation and required extracellular signal-regulated kinase (ERK)1/2 signalling and translation of pre-existing mRNAs. Once induced, the burst pattern persisted for several days; its maintenance phase (> 4 h) was dependent on gene transcription taking place in a critical period of 120 min following induction. Thus, cultured hippocampal neurones display a simple, transcription and protein synthesis-dependent form of plasticity. The non-invasive nature of MEA recordings provides a significant advantage over traditional assays for synaptic connectivity (i.e. long-term potentiation in brain slices) and facilitates the search for activity-regulated genes critical for late-phase plasticity.
Figures







References
-
- Alarcon JM, Malleret G, Touzani K, Vronskaya S, Ishii S, Kandel ER, Barco A. Chromatin acetylation, memory, and LTP are impaired in CBP+/– mice: a model for the cognitive deficit in Rubinstein–Taybi syndrome and its amelioration. Neuron. 2004;42:947–959. - PubMed
-
- Antonova I, Arancio O, Trillat AC, Wang HG, Zablow L, Udo H, Kandel ER, Hawkins RD. Rapid increase in clusters of presynaptic proteins at onset of long-lasting potentiation. Science. 2001;294:1547–1550. - PubMed
-
- Bacci A, Verderio C, Pravettoni E, Matteoli M. Synaptic and intrinsic mechanisms shape synchronous oscillations in hippocampal neurons in culture. Eur J Neurosci. 1999;11:389–397. - PubMed
-
- Bading H. Transcription-dependent neuronal plasticity: The nuclear calcium hypothesis. Eur J Biochem. 2000;267:5280–5283. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

