Length-dependent filament formation assessed from birefringence increases during activation of porcine tracheal muscle
- PMID: 15618272
- PMCID: PMC1665598
- DOI: 10.1113/jphysiol.2004.079822
Length-dependent filament formation assessed from birefringence increases during activation of porcine tracheal muscle
Abstract
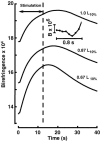
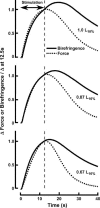
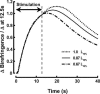
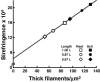
Birefringence and force produced by pig tracheal smooth muscles were recorded every 100 ms during electrically stimulated tetani at muscle lengths that varied 1.5-fold and at the peak of acetylcholine contractures at the same lengths. Isometric force was nearly the same at all lengths. Resting birefringence at the longest length was 30% greater than that at the shortest length. During tetani, birefringence increased with approximately the same time course as force, rising by 20% at the shortest length and 9% at the longest length, and continued to increase by an additional 0.5-1.5% of the resting value for 2-8 s after stimulation ended and force began to fall. This late increase was greatest and more sustained at longer lengths. During contractures, birefringence increased by 25 and 18% at the shortest and longest lengths, respectively. Comparison of these results with our published thick-filament densities suggests that thick-filament density increased by about 80, 72 and 50% during contractures at the short, intermediate and long lengths, and that approximately 35% of birefringence in the resting muscle at the longest length was not due to thick filaments. These findings support the hypotheses that tracheal smooth muscle adapts to longer lengths by increasing thick-filament mass and that myosin thick filaments are evanescent, dissociating partially during relaxation and reforming upon activation. The results further suggest that thick-filament formation is sufficiently rapid to account for the velocity slowing and some of the force increase observed during the rise of activation of tracheal smooth muscle.
Figures


References
-
- Barb MD, Morris AB, Maass-Moreno R, Ragozzino J, Ford LE. Versatile, high-speed force transducer using a laser diode as an optical lever. J Appl Physiol. 2000;88:308–314. - PubMed
-
- Bennett HS. Methods applicable to the study of both fresh and fixed materials. In: Jones RM, editor. Mcclung's Handbook of Microscopical Techniques. New York: Harper; 1950. pp. 591–677.
-
- Dillon PF, Aksoy MO, Driska SP, Murphy RA. Myosin phosphorylation and the cross-bridge cycle in arterial smooth muscle. Science. 1981;211:495–497. - PubMed
-
- Dillon PF, Murphy RA. Tonic force maintenance with reduced shortening velocity in arterial smooth muscles. Am J Physiol Cell Phhyysiol. 1982;242:C102–108. - PubMed
-
- Eberstein A, Rosenfalck A. Birefringence of isolated muscle fibres in twitch and tetanus. Acta Physiol Scand. 1963;57:144–166.
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

