Candidate interneurones mediating group I disynaptic EPSPs in extensor motoneurones during fictive locomotion in the cat
- PMID: 15618278
- PMCID: PMC1665583
- DOI: 10.1113/jphysiol.2004.076034
Candidate interneurones mediating group I disynaptic EPSPs in extensor motoneurones during fictive locomotion in the cat
Abstract
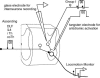
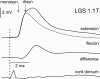
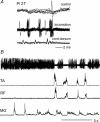
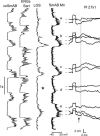
In the present study we sought to find interneurones responsible for the group I-evoked disynaptic excitation of hindlimb extensor motoneurones that occurs during fictive locomotion. Locomotion was produced by stimulation of the mesencephalic locomotor region (MLR) in decerebrate paralysed cats in which activation of ankle extensor group I afferents evoked a disynaptic excitation of motoneurones during the extension phase of fictive locomotion. Extracellular recordings were used to locate interneurones fulfilling all, or five of the six following criteria: (i) weak or no response to stimulation of extensor group I afferents in the absence of locomotion; (ii) strong group I activation during locomotion; (iii) group I activation at monosynaptic latencies; (iv) strong group I activation during only the extensor phase of locomotion; and (v) antidromic activation from the extensor motor nuclei; but (vi) no antidromic activation from rostral spinal segments. Candidate excitatory interneurones were located in mid to caudal parts of the L7 segments in areas where monosynaptic field potentials were evoked by group I afferents, within 2 mm of the stimulation site in the ventral horn from which they were antidromically activated. All were activated during extension by stimulation of group I afferents in extensor nerves. In the absence of peripheral nerve stimulation, six of the seven candidate excitatory interneurones were rhythmically active with maximal activation during the extension phase of fictive locomotion. Rhythmic activity during extension was also seen in five additional interneurones located near candidate interneurones but not activated by group I strength stimulation of the tested nerves. We suggest that the lumbosacral interneurones located in the intermediate laminae that can be activated by extensor group I afferents during the extension phase are a previously unknown population of interneurones, and may mediate group I-evoked disynaptic excitation of extensor motoneurones. Their rhythmic activity suggests that they also provide central excitatory drive to extensor motoneurones during locomotion.
Figures





References
-
- Conway BA, Hultborn H, Kiehn O. Proprioceptive input resets central locomotor rhythm in the spinal cord. Exp Brain Res. 1987;68:643–656. - PubMed
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Miscellaneous

