The cAMP binding domain: an ancient signaling module
- PMID: 15618393
- PMCID: PMC544069
- DOI: 10.1073/pnas.0408579102
The cAMP binding domain: an ancient signaling module
Abstract
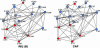
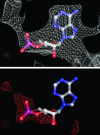
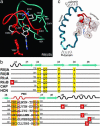
cAMP-binding domains from several different proteins were analyzed to determine the properties and interactions of this recognition motif. Systematic computational analyses, including structure-based sequence comparison, surface matching, affinity grid analysis, and analyses of the ligand protein interactions were carried out. These analyses show distinctive roles of the sugar phosphate and the adenine in the cAMP-binding module. We propose that the cAMP-binding regulatory proteins function by providing an allosteric system in which the presence or absence of cAMP produces a substantial structural change through the loss of hydrophobic interactions with the adenine ring and consequent repositioning of the C helix. The modified positioning of the helix in turn is recognized by a protein-binding event, completing the allostery.
Figures

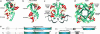


References
-
- Canaves, J. M. & Taylor, S. S. (2002) J. Mol. Evol. 54, 17-29. - PubMed
-
- Diller, T. C., Madhusudan, Xuong, N. H. & Taylor, S. S. (2001) Structure (London) 9, 73-82. - PubMed
-
- McKay, D. B. & Steitz, T. A. (1981) Nature 290, 744-749. - PubMed
-
- Weber, I. T., Steitz, T. A., Bubis, J. & Taylor, S. S. (1987) Biochemistry 26, 343-351. - PubMed
-
- Weber, I. T., Shabb, J. B. & Corbin, J. D. (1989) Biochemistry 28, 6122-6127. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

