A speed limit for conformational change of an allosteric membrane protein
- PMID: 15618401
- PMCID: PMC544059
- DOI: 10.1073/pnas.0406777102
A speed limit for conformational change of an allosteric membrane protein
Abstract
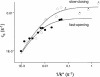
Neuromuscular acetylcholine receptors are synaptic ion channels that open and close with rate constants of approximately equal to 48,000 s(-1) and approximately equal to 1,700 s(-1), respectively (in adult mouse, at 24 degrees C, -100 mV membrane potential). Perturbations of many different sites in the protein can change these rate constants, with those in the extracellular domain mainly affecting channel-opening and many of those in the membrane and intracellular domains mainly affecting channel-closing. We used single-channel recordings to measure the total open time per activation (tau(b)) elicited by a low concentration of the natural transmitter, acetylcholine. tau(b) increased in constructs with mutations that increased the gating equilibrium constant by either increasing the opening or decreasing the closing rate constant. However, tau(b) did not approach the same asymptote in fast-opening and slow-closing constructs. The maximum value for the slow closers was about twice that for the fast openers. One interpretation of this difference is that there is an upper limit to the channel-opening rate constant, which we estimate to be approximately 0.86 mus(-1). One possibility is that this limit is the rate of conformational change in the absence of an overall activation barrier and thus reflects the kinetic prefactor for the acetylcholine receptor opening isomerization.
Figures

References
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

