Molecular diversity and regulation of renal potassium channels
- PMID: 15618483
- PMCID: PMC2838721
- DOI: 10.1152/physrev.00051.2003
Molecular diversity and regulation of renal potassium channels
Abstract
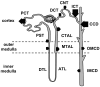
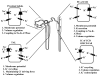
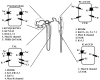
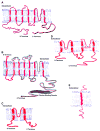
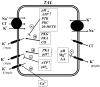
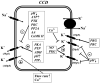
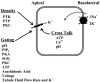
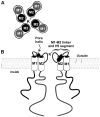
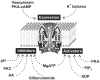
K(+) channels are widely distributed in both plant and animal cells where they serve many distinct functions. K(+) channels set the membrane potential, generate electrical signals in excitable cells, and regulate cell volume and cell movement. In renal tubule epithelial cells, K(+) channels are not only involved in basic functions such as the generation of the cell-negative potential and the control of cell volume, but also play a uniquely important role in K(+) secretion. Moreover, K(+) channels participate in the regulation of vascular tone in the glomerular circulation, and they are involved in the mechanisms mediating tubuloglomerular feedback. Significant progress has been made in defining the properties of renal K(+) channels, including their location within tubule cells, their biophysical properties, regulation, and molecular structure. Such progress has been made possible by the application of single-channel analysis and the successful cloning of K(+) channels of renal origin.
Figures


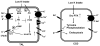







References
-
- Abbott GW, Butler MH, Bendahhou S, Dalakas MC, Ptacek LJ, Goldstein SA. MiRP2 forms potassium channels in skeletal muscle with Kv3.4 and its associated with periodic paralysis. Cell. 2001;104:217–231. - PubMed
-
- Abbott GW, Goldstein SA, Sesti F. Do all voltage-gated potassium channels use MiRPs? Circ Res. 2001;88:981–983. - PubMed
-
- Abbott GWS. MiRP1 forms potassium channels with HERG IKr and is associated with cardiac arrhythmia. Cell. 1999;97:175–187. - PubMed
-
- Adu D, Turney J, Michael J, McMaster P. Hyperkalaemia in cyclosporin-treated renal allograft recipients. Lancet. 1983;2:370–372. - PubMed
-
- Aguilar-Bryan L, Clement JP, Gonzalez G, Kunjilwar K, Babenko A, Bryan J. Toward understanding the assembly and structure of KATP channels. Physiol Rev. 1998;78:227–245. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- DK-17433/DK/NIDDK NIH HHS/United States
- DK-065172-01/DK/NIDDK NIH HHS/United States
- DK-54998/DK/NIDDK NIH HHS/United States
- R01 DK047402/DK/NIDDK NIH HHS/United States
- DK-54999/DK/NIDDK NIH HHS/United States
- P01 HL034300/HL/NHLBI NIH HHS/United States
- R01 DK065172/DK/NIDDK NIH HHS/United States
- HL-34300/HL/NHLBI NIH HHS/United States
- DK-47402/DK/NIDDK NIH HHS/United States
- R01 DK054999/DK/NIDDK NIH HHS/United States
- DK-54983/DK/NIDDK NIH HHS/United States
- R01 DK054998/DK/NIDDK NIH HHS/United States
- R56 DK054983/DK/NIDDK NIH HHS/United States
- R01 DK054983/DK/NIDDK NIH HHS/United States
- R01 DK048105/DK/NIDDK NIH HHS/United States
- DK-48105-08/DK/NIDDK NIH HHS/United States
- DK-17433-30/DK/NIDDK NIH HHS/United States
- P01 DK017433/DK/NIDDK NIH HHS/United States
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

