Neurexins induce differentiation of GABA and glutamate postsynaptic specializations via neuroligins
- PMID: 15620359
- PMCID: PMC2826211
- DOI: 10.1016/j.cell.2004.11.035
Neurexins induce differentiation of GABA and glutamate postsynaptic specializations via neuroligins
Abstract
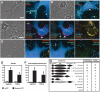
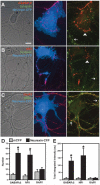
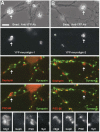
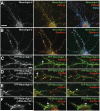
Formation of synaptic connections requires alignment of neurotransmitter receptors on postsynaptic dendrites opposite matching transmitter release sites on presynaptic axons. beta-neurexins and neuroligins form a trans-synaptic link at glutamate synapses. We show here that neurexin alone is sufficient to induce glutamate postsynaptic differentiation in contacting dendrites. Surprisingly, neurexin also induces GABA postsynaptic differentiation. Conversely, neuroligins induce presynaptic differentiation in both glutamate and GABA axons. Whereas neuroligins-1, -3, and -4 localize to glutamate postsynaptic sites, neuroligin-2 localizes primarily to GABA synapses. Direct aggregation of neuroligins reveals a linkage of neuroligin-2 to GABA and glutamate postsynaptic proteins, but the other neuroligins only to glutamate postsynaptic proteins. Furthermore, mislocalized expression of neuroligin-2 disperses postsynaptic proteins and disrupts synaptic transmission. Our findings indicate that the neurexin-neuroligin link is a core component mediating both GABAergic and glutamatergic synaptogenesis, and differences in isoform localization and binding affinities may contribute to appropriate differentiation and specificity.
Figures


References
-
- Biederer T, Sudhof TC. Mints as adaptors. Direct binding to neurexins and recruitment of munc18. J. Biol. Chem. 2000;275:39803–39806. - PubMed
-
- Biederer T, Sara Y, Mozhayeva M, Atasoy D, Liu X, Kavalali ET, Sudhof TC. SynCAM, a synaptic adhesion molecule that drives synapse assembly. Science. 2002;297:1525–1531. - PubMed
-
- Craig AM, Boudin H. Molecular heterogeneity of central synapses: afferent and target regulation. Nat. Neurosci. 2001;4:569–578. - PubMed
-
- Dalva MB, Takasu MA, Lin MZ, Shamah SM, Hu L, Gale NW, Greenberg ME. EphB receptors interact with NMDA receptors and regulate excitatory synapse formation. Cell. 2000;103:945–956. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

