The endogenous peptide apelin potently improves cardiac contractility and reduces cardiac loading in vivo
- PMID: 15621035
- PMCID: PMC2517138
- DOI: 10.1016/j.cardiores.2004.08.018
The endogenous peptide apelin potently improves cardiac contractility and reduces cardiac loading in vivo
Abstract
Objective: The endogenous peptide apelin is differentially regulated in cardiovascular disease but the nature of its role in cardiac function remains unclear.
Methods: We investigated the functional relevance of this peptide using ECG and respiration gated magnetic resonance imaging, conductance catheter pressure-volume hemodynamic measurements, and echocardiography in vivo. In addition, we carried out histology and immunohistochemistry to assess cardiac hypertrophy and to localize apelin and APJ in the adult and embryonic mouse heart.
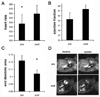
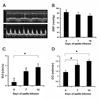
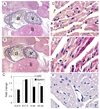
Results: Intraperitoneal injection of apelin (300 microg/kg) resulted in a decrease in left ventricular end diastolic area (pre: 0.122+/-0.007; post: 0.104+/-0.005 cm(2), p=0.006) and an increase in heart rate (pre: 537+/-20; post: 559+/-19 beats per minute, p=0.03). Hemodynamic measurements revealed a marked increase in ventricular elastance (pre: 3.7+/-0.9; post: 6.5+/-1.4 mm Hg/RVU, p=0.018) and preload recruitable stroke work (pre: 27.4+/-8.0; post: 51.8+/-3.1, p=0.059) with little change in diastolic parameters following acute infusion of apelin. Chronic infusion (2 mg/kg/day) resulted in significant increases in the velocity of circumferential shortening (baseline: 5.36+/-0.401; 14 days: 6.85+/-0.358 circ/s, p=0.049) and cardiac output (baseline: 0.142+/-0.019; 14 days: 0.25+/-0.019 l/min, p=0.001) as determined by 15 MHz echocardiography. Post-mortem corrected heart weights were not different between apelin and saline groups (p=0.5) and histology revealed no evidence of cellular hypertrophy in the apelin group (nuclei per unit area, p=0.9). Immunohistochemistry studies revealed APJ staining of myocardial cells in all regions of the adult mouse heart. Antibody staining, as well as quantitative real time polymerase chain reaction identified expression of both APJ and apelin in embryonic myocardium as early as embryonic day 13.5.
Conclusions: Apelin reduces left ventricular preload and afterload and increases contractile reserve without evidence of hypertrophy. These results associate apelin with a positive hemodynamic profile and suggest it as an attractive target for pharmacotherapy in the setting of heart failure.
Figures

Comment in
-
On the cardiovascular activity of apelin.Cardiovasc Res. 2005 Jan 1;65(1):8-9. doi: 10.1016/j.cardiores.2004.10.027. Cardiovasc Res. 2005. PMID: 15621027 No abstract available.
References
-
- Chen MM, Ashley EA, Deng DX, Tsalenko A, Deng A, Tabibiazar R, et al. Novel Role for the Potent Endogenous Inotrope Apelin in Human Cardiac Dysfunction. Circulation. 2003;108:1432–1439. - PubMed
-
- O'Dowd BF, Heiber M, Chan A, Heng HH, Tsui LC, Kennedy JL, et al. A human gene that shows identity with the gene encoding the angiotensin receptor is located on chromosome 11. Gene. 1993;136:355–360. - PubMed
-
- Tatemoto K, Hosoya M, Habata Y, Fujii R, Kakegawa T, Zou MX, et al. Isolation and characterization of a novel endogenous peptide ligand for the human APJ receptor. Biochem Biophys Res Commun. 1998;251:471–476. - PubMed
-
- Kawamata Y, Habata Y, Fukusumi S, Hosoya M, Fujii R, Hinuma S, et al. Molecular properties of apelin: tissue distribution and receptor binding. Biochim Biophys Acta. 2001;1538:162–171. - PubMed
-
- Foldes G, Horkay F, Szokodi I, Vuolteenaho O, Ilves M, Lindstedt KA, et al. Circulating and cardiac levels of apelin, the novel ligand of the orphan receptor APJ, in patients with heart failure. Biochem Biophys Res Commun. 2003;308:480–485. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases

