Acquired deficit of forebrain glucocorticoid receptor produces depression-like changes in adrenal axis regulation and behavior
- PMID: 15623560
- PMCID: PMC544280
- DOI: 10.1073/pnas.0406458102
Acquired deficit of forebrain glucocorticoid receptor produces depression-like changes in adrenal axis regulation and behavior
Abstract
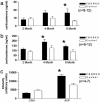
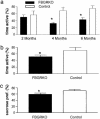
Dysregulation of the hypothalamic-pituitary-adrenal (HPA) axis is a hallmark of major depressive disorder. A number of studies have shown that this dysregulation is correlated with impaired forebrain glucocorticoid receptor (GR) function. To determine whether a primary, acquired deficit in forebrain GR signaling is an etiologic factor in the pathogenesis of depression, we generated a line of mice with time-dependent, forebrain-specific disruption of GR (FBGRKO). These mice develop a number of both physiological and behavioral abnormalities that mimic major depressive disorder in humans, including hyperactivity of the HPA axis, impaired negative feedback regulation of the HPA axis and, increased depression-like behavior. Importantly, a number of these abnormalities are normalized by chronic treatment with the tricyclic antidepressant, imipramine. Our findings suggest that imipramine's proposed activities on forebrain GR function are not essential for its antidepressant effects, and that alteration in GR expression may play a causative role in disease onset of major depressive disorder.
Figures



References
-
- Herman, J. P., Figueiredo, H., Mueller, N. K., Ulrich-Lai, Y., Ostrander, M. M., Choi, D. C. & Cullinan, W. E. (2003) Front. Neuroendocrinol. 24, 151-180. - PubMed
-
- O'Toole, S. M., Sekula, L. K. & Rubin, R. T. (1997) Biol. Psychiatry 42, 85-89. - PubMed
-
- Calfa, G., Kademian, S., Ceschin, D., Vega, G., Rabinovich, G. A. & Volosin, M. (2003) Psychoneuroendocrinology 28, 687-701. - PubMed
-
- Gass, P., Reichardt, H. M., Strekalova, T., Henn, F. & Tronche, F. (2001) Physiol. Behav. 73, 811-825. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases

