Acute ENaC stimulation by cAMP in a kidney cell line is mediated by exocytic insertion from a recycling channel pool
- PMID: 15623897
- PMCID: PMC2217480
- DOI: 10.1085/jgp.200409124
Acute ENaC stimulation by cAMP in a kidney cell line is mediated by exocytic insertion from a recycling channel pool
Abstract
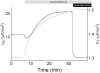
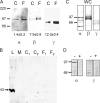
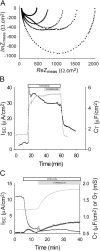
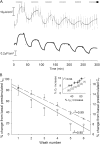
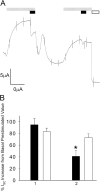
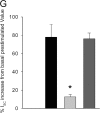
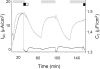
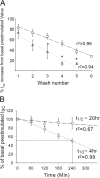
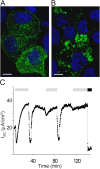
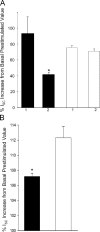
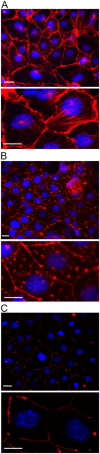
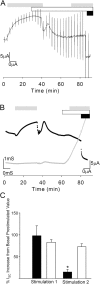
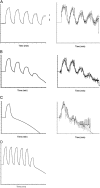
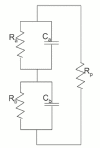
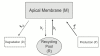
Acute hormonal regulation of the epithelial sodium channel (ENaC) in tight epithelia increases transcellular Na(+) transport via trafficking of intracellular channels to the apical surface. The fate of the channels removed from the apical surface following agonist washout is less clear. By repetitively stimulating polarized mouse cortical collecting duct (mCCD, (MPK)CCD(14)) epithelia, we evaluated the hypothesis that ENaC recycles through an intracellular pool to be available for reinsertion into the apical membrane. Short circuit current (I(SC)), membrane capacitance (C(T)), and conductance (G(T)) were recorded from mCCD epithelia mounted in modified Ussing chambers. Surface biotinylation of ENaC demonstrated an increase in channel number in the apical membrane following cAMP stimulation. This increase was accompanied by a 83 +/- 6% (n = 31) increase in I(SC) and a 15.3 +/- 1.5% (n = 15) increase in C(T). Selective membrane permeabilization demonstrated that the C(T) increase was due to an increase in apical membrane capacitance. I(SC) and C(T) declined to basal levels on stimulus washout. Repetitive cAMP stimulation and washout (approximately 1 h each cycle) resulted in response fatigue; DeltaI(SC) decreased approximately 10% per stimulation-recovery cycle. When channel production was blocked by cycloheximide, DeltaI(SC) decreased approximately 15% per stimulation cycle, indicating that newly synthesized ENaC contributed a relatively small fraction of the channels mobilized to the apical membrane. Selective block of surface ENaC by benzamil demonstrated that channels inserted from a subapical pool made up >90% of the stimulated I(SC), and that on restimulation a large proportion of channels retrieved from the apical surface were reinserted into the apical membrane. Channel recycling was disrupted by brefeldin A, which inhibited ENaC exocytosis, by chloroquine, which inhibited ENaC endocytosis and recycling, and by latrunculin A, which blocked ENaC exocytosis. A compartment model featuring channel populations in the apical membrane and intracellular recycling pool provided an adequate kinetic description of the I(SC) responses to repetitive stimulation. The model supports the concept of ENaC recycling in response to repetitive cAMP stimulation.
Figures


References
-
- Benos, D.J., M.S. Awayda, I.I. Ismailov, and J.P. Johnson. 1995. Structure and function of amiloride-sensitive Na+ channels. J. Membr. Biol. 143:1–18. - PubMed
-
- Bradford, A.L., I.I. Ismailov, J.M. Achard, D.G. Warnock, J.K. Bubien, and D.J. Benos. 1995. Immunopurification and functional reconstitution of a Na+ channel complex from rat lymphocytes. Am. J. Physiol. 269:C601–C611. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

