Induction, differentiation, and remodeling of blood vessels after transplantation of Bcl-2-transduced endothelial cells
- PMID: 15625106
- PMCID: PMC544288
- DOI: 10.1073/pnas.0408357102
Induction, differentiation, and remodeling of blood vessels after transplantation of Bcl-2-transduced endothelial cells
Abstract
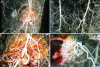
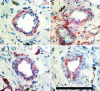
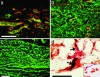
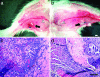
Implants of collagen-fibronectin gels containing Bcl-2-transduced human umbilical vein endothelial cells (Bcl-2-HUVECs) induce the formation of human endothelial cell (EC)/murine vascular smooth muscle cell (VSMC) chimeric vessels in immunodeficient mice. Microfil casting of the vasculature 60 d after implantation reveals highly branched microvascular networks within the implants that connect with and induce remodeling of conduit vessels arising from the abdominal wall circulation. Approximately 85% of vessels within the implants are lined by Bcl-2-positive human ECs expressing VEGFR1, VEGFR2, and Tie-2, but not integrin alpha(v)beta(3). The human ECs are seated on a well formed human laminin/collagen IV-positive basement membrane, and are surrounded by mouse VSMCs expressing SM-alpha actin, SM myosin, SM22alpha, and calponin, all markers of contractile function. Transmission electron microscopy identified well formed EC-EC junctions, chimeric arterioles with concentric layers of contractile VSMC, chimeric capillaries surrounded by pericytes, and chimeric venules. Bcl-2-HUVEC-lined vessels retain 70-kDa FITC-dextran, but not 3-kDa dextran; local histamine rapidly induces leak of 70-kDa FITC-dextran or India ink. As in skin, TNF induces E-selectin and vascular cell adhesion molecule 1 only on venular ECs, whereas intercellular adhesion molecule-1 is up-regulated on all human ECs. Bcl-2-HUVEC implants are able to engraft within and increase perfusion of ischemic mouse gastrocnemius muscle after femoral artery ligation. These studies show that cultured Bcl-2-HUVECs can differentiate into arterial, venular, and capillary-like ECs when implanted in vivo, and induce arteriogenic remodeling of the local mouse vessels. Our results support the utility of differentiated EC transplantation to treat tissue ischemia.
Figures


References
-
- Jain, R. K. (2003) Nat. Med. 9, 685-693. - PubMed
-
- Choi, J., Enis, D., Koh, K., Shiao, S. & Pober, J. S. (2004) Annu. Rev. Immunol. 22, 683-709. - PubMed
-
- Tsurumi, Y., Takeshita, S., Chen, D., Kearney, M., Rossow, S. T., Passeri, J., Horowitz, J. R., Symes, J. F. & Isner, J. M. (1996) Circulation 94, 3281-3290. - PubMed
-
- Dor, Y., Djonov, V. & Keshet, E. (2003) Ann. N.Y. Acad. Sci. 995, 208-216. - PubMed
-
- de Muinck, E. D. & Simons, M. (2004) J. Mol. Cell. Cardiol. 36, 25-32. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

