Role of glycosylation and membrane environment in nicotinic acetylcholine receptor stability
- PMID: 15626708
- PMCID: PMC1305231
- DOI: 10.1529/biophysj.104.052944
Role of glycosylation and membrane environment in nicotinic acetylcholine receptor stability
Abstract
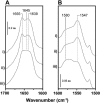
The effects of glycosylation and membrane environment on the structural stability of the nicotinic acetylcholine receptor (nAChR) from Torpedo have been investigated to improve our understanding of factors that influence eukaryotic membrane protein crystallization. Gel shift assays and carbohydrate-specific staining show that the deglycosylation enzyme, Endo F1, removes at least 50% of membrane-reconstituted nAChR glycosylation. The extent of deglycosylation with Endo F1 increases upon detergent solubilization. Removal of between 60-100% of high mannose moieties from the nAChR has no effect on nAChR secondary structure, stability, or flexibility. Deglycosylation does not influence either agonist binding or the ability of the nAChR to undergo agonist-induced conformational change. In contrast, nAChR structural stability, flexibility, and function are all negatively influenced by simple changes in reconstituted membrane lipid composition. Our results suggest that deglycosylation may represent a feasible approach for enhancing the crystallizability of the nAChR. Our data also demonstrate that the dependence of nAChR structural stability on lipid environment may represent a significant obstacle to nAChR crystallization. Some membrane proteins may have evolved complex interactions with their lipid environments. Understanding the complexity of these interactions may be essential for devising an appropriate strategy for the crystallization of some membrane proteins.
Figures



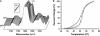

References
-
- Anholt, R., J. Lindstrom, and M. Montal., 1980. Functional equivalence of monomeric and dimeric forms of purified acetylcholine receptors from Torpedo californica in reconstituted lipid vesicles. Eur. J. Biochem. 109:481–487. - PubMed
-
- Baenziger, J. E., T. E. Darsaut, and M. L. Morris. 1999. Internal dynamics of the nicotinic acetylcholine receptor in reconstituted membranes. Biochemistry. 38:4905–4911. - PubMed
-
- Baenziger, J. E., M. L. Morris, T. E. Darsaut, and S. E. Ryan. 2000. Effect of membrane lipid composition on the conformational equilibria of the nicotinic acetylcholine receptor. J. Biol. Chem. 275:777–784. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

