Surface expression of Hsp25 and Hsp72 differentially regulates tumor growth and metastasis
- PMID: 15627887
- PMCID: PMC1764489
- DOI: 10.1159/000081387
Surface expression of Hsp25 and Hsp72 differentially regulates tumor growth and metastasis
Abstract
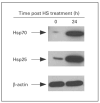
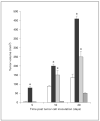
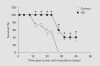
The expression of unique surface structures on tumors that allow for recognition and activation of host immunocompetent cells plays an important role in determining tumor growth and/or metastasis. Recent studies have identified an important role for heat shock proteins (Hsp) in antitumor surveillance; however, the exact role of Hsp expressed on the surface of tumors has not been fully addressed. In this study, we show that 4T1 mammary adenocarcinoma cells sorted for high Hsp25 surface expression (Hsp25(high)) grow significantly faster than cells sorted for intermediate Hsp25 surface expression (Hsp25(intermediate)) or wild-type 4T1 cells implanted into the abdominal breast gland of female BALB/c mice (p < 0.05). In addition, histological examination of lung tissues revealed that Hsp25(high) 4T1 cells metastasized to the lungs more aggressively than either Hsp25(intermediate) or wild-type 4T1 cells (p < 0.05). Exposure of 4T1 cells to nonlethal heat shock (43 degrees C, 30 min) induced the surface expression of Hsp72 and a concomitant reduction in Hsp25 surface expression. The growth and metastastic potential of Hsp72(+) 4T1 cells was significantly less than that of Hsp25(high), Hsp25(intermediate) or wild-type 4T1 cells (p < 0.05). Taken together, these studies identify an important role for expression of Hsp25 and Hsp72 during tumor growth and metastatic spread which might be helpful in the design of antimetastatic therapies.
Copyright 2004 S. Karger AG, Basel.
Figures



References
-
- Craig EA, Gross CA. Is hsp70 the cellular thermometer? Trends Biochem Sci. 1991;16:135–140. - PubMed
-
- Lindquist S, Craig EA. The heat-shock proteins. Annu Rev Genet. 1988;22:631–677. - PubMed
-
- Morimoto RI, Tissières A, Georgopoulos C. The Biology of Heat Shock Proteins and Molecular Chaperones. Cold Spring Harbor: Cold Spring Harbor Laboratory Press; 1994.
-
- Ciocca DR, Green S, Elledge RM, Clark GM, Pugh R, Ravdin P, Lew D, Martino S, Osborne CK. Heat shock proteins hsp27 and hsp70: Lack of correlation with response to tamoxifen and clinical course of disease in estrogen receptor-positive metastatic breast cancer (a Southwest Oncology Group Study) Clin Cancer Res. 1998;4:1263–1266. - PubMed
-
- Ciocca DR, Vargas-Roig LM. Hsp27 as a prognostic and predictive factor in cancer. Prog Mol Subcell Biol. 2002;28:205–218. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

