Intimin-mediated export of passenger proteins requires maintenance of a translocation-competent conformation
- PMID: 15629924
- PMCID: PMC543525
- DOI: 10.1128/JB.187.2.522-533.2005
Intimin-mediated export of passenger proteins requires maintenance of a translocation-competent conformation
Abstract
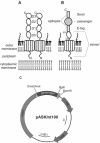
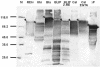
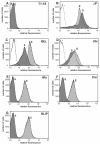
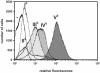
Intimins from pathogenic bacteria promote intimate bacterial adhesion to epithelial cells. Several structurally similar domains form on the bacterial cell surface an extended rigid rod that exposes the carboxy-terminal domain, which interacts with the translocated intimin receptor. We constructed a series of intimin-derived fusion proteins consisting of carboxy-terminally truncated intimin and the immunoglobulin light-chain variable domain REIv, ubiquitin, calmodulin, beta-lactamase inhibitor protein, or beta-lactamase. By systematically investigating the intimin-mediated cell surface exposure of these passenger domains in the presence or absence of compounds that interfere with outer membrane stability or passenger domain folding, we acquired experimental evidence that intimin-mediated protein export across the outer membrane requires, prior to export, the maintenance of a translocation-competent conformation that may be distinct from the final protein structure. We propose that, during export, competition exists between productive translocation and folding of the passenger domain in the periplasm into a stable conformation that is not compatible with translocation through the bacterial outer membrane. These results may expand understanding of the mechanism by which intimins are inserted into the outer membrane and expose extracellular domains on the cell surface.
Figures

References
-
- Akiyama, Y., S. Kamitani, N. Kusukawa, and K. Ito. 1992. In vitro catalysis of oxidative folding of disulfide-bonded proteins by the Escherichia coli dsbA (ppfA) gene product. J. Biol. Chem. 267:22440-22445. - PubMed
-
- Bardwell, J. C., K. McGovern, and J. Beckwith. 1991. Identification of a protein required for disulfide bond formation in vivo. Cell 67:581-589. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

