Noncanonical Wnt signaling regulates midline convergence of organ primordia during zebrafish development
- PMID: 15630025
- PMCID: PMC540234
- DOI: 10.1101/gad.1253605
Noncanonical Wnt signaling regulates midline convergence of organ primordia during zebrafish development
Abstract
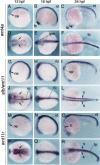
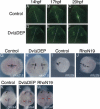
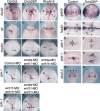
Several components of noncanonical Wnt signaling pathways are involved in the control of convergence and extension (CE) movements during zebrafish and Xenopus gastrulation. However, the complexity of these pathways and the wide patterns of expression and activity displayed by some of their components immediately suggest additional morphogenetic roles beyond the control of CE. Here we show that the key modular intracellular mediator Dishevelled, through a specific activation of RhoA GTPase, controls the process of convergence of endoderm and organ precursors toward the embryonic midline in the zebrafish embryo. We also show that three Wnt noncanonical ligands wnt4a, silberblick/wnt11, and wnt11-related regulate this process by acting in a largely redundant way. The same ligands are also required, nonredundantly, to control specific aspects of CE that involve interaction of Dishevelled with mediators different from that of RhoA GTPase. Overall, our results uncover a late, previously unexpected role of noncanonical Wnt signaling in the control of midline assembly of organ precursors during vertebrate embryo development.
Figures


References
-
- Alexander J., Rothenberg, M., Henry, G.L., and Stainier, D.Y. 1999. casanova plays an early and essential role in endoderm formation in zebrafish. Dev. Biol. 215: 343–357. - PubMed
-
- Bakkers J., Kramer, C., Pothof, J., Quaedvlieg, N.E., Spaink, H.P., and Hammerschmidt, M. 2004. Has2 is required upstream of Rac1 to govern dorsal migration of lateral cells during zebrafish gastrulation. Development 131: 525–537. - PubMed
-
- Buckles G.R., Thorpe, C.J., Ramel, M.C., and Lekven, A.C. 2004. Combinatorial Wnt control of zebrafish midbrain-hindbrain boundary formation. Mech. Dev. 121: 437–447. - PubMed
-
- Carachi R. and Azmy, A. 2002. Foregut duplications. Pediatr. Surg. Int. 18: 371–374. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
