The Plasmodium circumsporozoite protein is proteolytically processed during cell invasion
- PMID: 15630135
- PMCID: PMC1995445
- DOI: 10.1084/jem.20040989
The Plasmodium circumsporozoite protein is proteolytically processed during cell invasion
Abstract
The circumsporozoite protein (CSP) is the major surface protein of Plasmodium sporozoites, the infective stage of malaria. Although CSP has been extensively studied as a malaria vaccine candidate, little is known about its structure. Here, we show that CSP is proteolytically cleaved by a papain family cysteine protease of parasite origin. Our data suggest that the highly conserved region I, found just before the repeat region, contains the cleavage site. Cleavage occurs on the sporozoite surface when parasites contact target cells. Inhibitors of CSP processing inhibit cell invasion in vitro, and treatment of mice with E-64, a highly specific cysteine protease inhibitor, completely inhibits sporozoite infectivity in vivo.
Figures


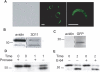
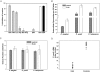
References
-
- Sinnis, P., and E. Nardin. 2002. Sporozoite antigens: biology and immunology of the circumsporozoite protein and thrombospondin related anonymous protein. Malaria Immunology. P. Perlmann and M. Troye-Blomberg, editors. S. Karger Press, Basel, Switzerland. pp. 70–96. - PubMed
-
- Menard, R., A.A. Sultan, C. Cortes, R. Altszuler, M.R. van Dijk, C.J. Janse, A.P. Waters, R.S. Nussenzweig, and V. Nussenzweig. 1997. Circumsporozoite protein is required for development of malaria sporozoites in mosquitoes. Nature. 385:336–340. - PubMed
-
- Goundis, D., and K.B.M. Reid. 1988. Properdin, the terminal complement components, thrombospondin and the circumsporozoite protein of malaria parasites contain similar sequence motifs. Nature. 335:82–85. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

