Hypoxia-induced neutrophil survival is mediated by HIF-1alpha-dependent NF-kappaB activity
- PMID: 15630139
- PMCID: PMC2212759
- DOI: 10.1084/jem.20040624
Hypoxia-induced neutrophil survival is mediated by HIF-1alpha-dependent NF-kappaB activity
Abstract
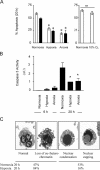
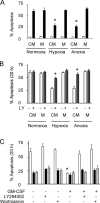
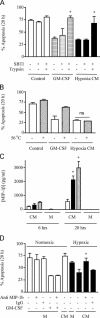
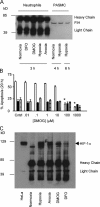
Neutrophils are key effector cells of the innate immune response and are required to migrate and function within adverse microenvironmental conditions. These inflammatory sites are characterized by low levels of oxygen and glucose and high levels of reductive metabolites. A major regulator of neutrophil functional longevity is the ability of these cells to undergo apoptosis. We examined the mechanism by which hypoxia causes an inhibition of neutrophil apoptosis in human and murine neutrophils. We show that neutrophils possess the hypoxia-inducible factor (HIF)-1alpha and factor inhibiting HIF (FIH) hydroxylase oxygen-sensing pathway and using HIF-1alpha-deficient myeloid cells demonstrate that HIF-1alpha is directly involved in regulating neutrophil survival in hypoxia. Gene array, TaqMan PCR, Western blotting, and oligonucleotide binding assays identify NF-kappaB as a novel hypoxia-regulated and HIF-dependent target, with inhibition of NF-kappaB by gliotoxin or parthenolide resulting in the abrogation of hypoxic survival. In addition, we identify macrophage inflammatory protein-1beta as a novel hypoxia-induced neutrophil survival factor.
Figures
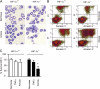
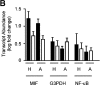

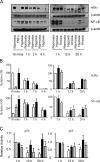
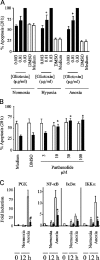
References
-
- Nunn, J.F. 1993. Nunn's Applied Respiratory Physiology. Cambridge University Press, Cambridge, UK. 658 pp.
-
- Ellis, G.A., S.E. Edmonds, K. Gaffney, R.B. Williams, and D. Blake. 1994. Synovial tissue oxygenation profile in inflamed and non-inflamed knee joints. Br. J. Rheumatol. 33:172.
-
- Niinikoski, J., T.K. Hunt, and J. Englebert Dunphy. 1972. Oxygen supply in healing tissue. Am. J. Surg. 123:247–252. - PubMed
-
- Levene, P.A., and G.M. Meyer. 1912. The action of leucocytes on glucose. J. Biol. Chem. 11:361–370.
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Miscellaneous

