Distinct organ-specific metastatic potential of individual breast cancer cells and primary tumors
- PMID: 15630443
- PMCID: PMC539194
- DOI: 10.1172/JCI22320
Distinct organ-specific metastatic potential of individual breast cancer cells and primary tumors
Abstract
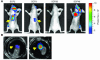
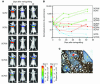
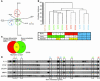
We used bioluminescence imaging to reveal patterns of metastasis formation by human breast cancer cells in immunodeficient mice. Individual cells from a population established in culture from the pleural effusion of a breast cancer patient showed distinct patterns of organ-specific metastasis. Single-cell progenies derived from this population exhibited markedly different abilities to metastasize to the bone, lung, or adrenal medulla, which suggests that metastases to different organs have different requirements. Transcriptomic profiling revealed that these different single-cell progenies similarly express a previously described "poor-prognosis" gene expression signature. Unsupervised classification using the transcriptomic data set supported the hypothesis that organ-specific metastasis by breast cancer cells is controlled by metastasis-specific genes that are separate from a general poor-prognosis gene expression signature. Furthermore, by using a gene expression signature associated with the ability of these cells to metastasize to bone, we were able to distinguish primary breast carcinomas that preferentially metastasized to bone from those that preferentially metastasized elsewhere. These results suggest that the bone-specific metastatic phenotypes and gene expression signature identified in a mouse model may be clinically relevant.
Figures





References
-
- Fidler IJ. The pathogenesis of cancer metastasis: the ‘seed and soil’ hypothesis revisited. Nat. Rev. Cancer. 2003;3:453–458. - PubMed
-
- Chambers AF, Groom AC, MacDonald IC. Dissemination and growth of cancer cells in metastatic sites. Nat. Rev. Cancer. 2002;2:563–572. - PubMed
-
- Tarin D, Vass AC, Kettlewell MG, Price JE. Absence of metastatic sequelae during long-term treatment of malignant ascites by peritoneo-venous shunting. A clinico-pathological report. Invasion Metastasis. 1984;4:1–12. - PubMed
-
- Fidler IJ. Metastasis: quantitative analysis of distribution and fate of tumor emboli labeled with 125 I-5-iodo-2′-deoxyuridine. J. Natl. Cancer Inst. 1970;45:773–782. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

