Oncogenic AKAP9-BRAF fusion is a novel mechanism of MAPK pathway activation in thyroid cancer
- PMID: 15630448
- PMCID: PMC539203
- DOI: 10.1172/JCI23237
Oncogenic AKAP9-BRAF fusion is a novel mechanism of MAPK pathway activation in thyroid cancer
Abstract
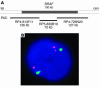
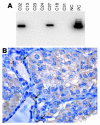
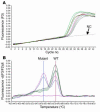
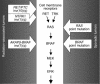
Genes crucial for cancer development can be mutated via various mechanisms, which may reflect the nature of the mutagen. In thyroid papillary carcinomas, mutations of genes coding for effectors along the MAPK pathway are central for transformation. BRAF point mutation is most common in sporadic tumors. By contrast, radiation-induced tumors are associated with paracentric inversions activating the receptor tyrosine kinases RET and NTRK1. We report here a rearrangement of BRAF via paracentric inversion of chromosome 7q resulting in an in-frame fusion between exons 1-8 of the AKAP9 gene and exons 9-18 of BRAF. The fusion protein contains the protein kinase domain and lacks the autoinhibitory N-terminal portion of BRAF. It has elevated kinase activity and transforms NIH3T3 cells, which provides evidence, for the first time to our knowledge, of in vivo activation of an intracellular effector along the MAPK pathway by recombination. The AKAP9-BRAF fusion was preferentially found in radiation-induced papillary carcinomas developing after a short latency, whereas BRAF point mutations were absent in this group. These data indicate that in thyroid cancer, radiation activates components of the MAPK pathway primarily through chromosomal paracentric inversions, whereas in sporadic forms of the disease, effectors along the same pathway are activated predominantly by point mutations.
Figures



Comment in
-
A new mechanism of BRAF activation in human thyroid papillary carcinomas.J Clin Invest. 2005 Jan;115(1):20-3. doi: 10.1172/JCI23987. J Clin Invest. 2005. PMID: 15630436 Free PMC article.
References
-
- Kimura ET, et al. High prevalence of BRAF mutations in thyroid cancer: genetic evidence for constitutive activation of the RET/PTC-RAS-BRAF signaling pathway in papillary thyroid carcinoma. Cancer Res. 2003;63:1454–1457. - PubMed
-
- Soares P, et al. BRAF mutations and RET/PTC rearrangements are alternative events in the etiopathogenesis of PTC. Oncogene. 2003;22:4578–4580. - PubMed
-
- Frattini M, et al. Alternative mutations of BRAF, RET and NTRK1 are associated with similar but distinct gene expression patterns in papillary thyroid cancer. Oncogene. 2004;23:7436–7440. - PubMed
-
- Takahashi M, Ritz J, Cooper GM. Activation of a novel human transforming gene, ret, by DNA rearrangement. Cell. 1985;42:581–588. - PubMed
-
- Takahashi M. Structure and expression of the ret transforming gene. IARC Sci. Publ. 1988;1988:189–197. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Research Materials

