Endothelial lipase provides an alternative pathway for FFA uptake in lipoprotein lipase-deficient mouse adipose tissue
- PMID: 15630456
- PMCID: PMC539186
- DOI: 10.1172/JCI15972
Endothelial lipase provides an alternative pathway for FFA uptake in lipoprotein lipase-deficient mouse adipose tissue
Abstract
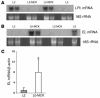
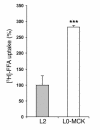
Lipoprotein lipase (LPL) is thought to be the only enzyme responsible for the catabolism of triglycerides (TGs) associated with TG-rich lipoproteins in adipose tissue (AT). However, LPL deficiency in humans and induced mutant mice is not associated with decreased fat mass. We investigated whether endothelial lipase (EL), a recently discovered phospholipase, might represent an alternative mechanism for the uptake of phospholipid-derived fatty acids in murine lipoprotein-deficient AT. When LPL was expressed in AT and isolated murine adipocytes, EL mRNA was not detectable. In contrast, mouse AT and isolated adipocytes that lacked LPL expressed large amounts of EL mRNA. The cellular phospholipase activity in LPL-deficient fat pads was increased 4-fold compared with control fat pads and could be inhibited to control levels by a specific EL antibody. Fatty acids produced by EL activity were absorbed by adipocytes and incorporated into the TG moiety of AT. Our results suggest that EL activity in AT and other peripheral tissues might contribute to the tissue uptake of free fatty acids, which could have important implications for the metabolism of plasma lipoproteins.
Figures




References
-
- Goldberg IJ. Lipoprotein lipase and lipolysis: central roles in lipoprotein metabolism and atherogenesis. J. Lipid Res. 1996;37:693–707. - PubMed
-
- Zechner R. The tissue-specific expression of lipoprotein lipase: implications for energy and lipoprotein metabolism. Curr. Opin. Lipidol. 1997;8:77–88. - PubMed
-
- Murthy V, Julien P, Gagne C. Molecular pathobiology of the human lipoprotein lipase gene. Pharmacol. Ther. 1996;70:101–135. - PubMed
-
- Ginzinger DG, et al. Lipid and lipoprotein analysis of cats with lipoprotein lipase deficiency. Eur. J. Clin. Invest. 1999;29:17–26. - PubMed
-
- Savonen R, et al. Chylomicron metabolism in an animal model for hyperlipoproteinemia type I. J. Lipid Res. 1999;40:1336–1346. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Miscellaneous

