LEA proteins prevent protein aggregation due to water stress
- PMID: 15631617
- PMCID: PMC1186703
- DOI: 10.1042/BJ20041931
LEA proteins prevent protein aggregation due to water stress
Abstract
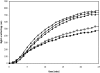
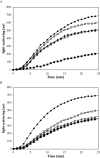
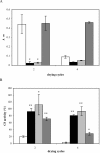
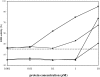
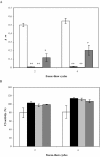
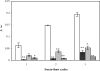
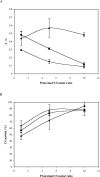
LEA (late embryogenesis abundant) proteins in both plants and animals are associated with tolerance to water stress resulting from desiccation and cold shock. However, although various functions of LEA proteins have been proposed, their precise role has not been defined. Recent bioinformatics studies suggest that LEA proteins might behave as molecular chaperones, and the current study was undertaken to test this hypothesis. Recombinant forms of AavLEA1, a group 3 LEA protein from the anhydrobiotic nematode Aphelenchus avenae, and Em, a group 1 LEA protein from wheat, have been subjected to functional analysis. Heat-stress experiments with citrate synthase, which is susceptible to aggregation at high temperatures, suggest that LEA proteins do not behave as classical molecular chaperones, but they do exhibit a protective, synergistic effect in the presence of the so-called chemical chaperone, trehalose. In contrast, both LEA proteins can independently protect citrate synthase from aggregation due to desiccation and freezing, in keeping with a role in water-stress tolerance; similar results were obtained with lactate dehydrogenase. This is the first evidence of anti-aggregation activity of LEA proteins due to water stress. Again, a synergistic effect of LEA and trehalose was observed, which is significant given that non-reducing disaccharides are known to accumulate during dehydration in plants and nematodes. A model is proposed whereby LEA proteins might act as a novel form of molecular chaperone, or 'molecular shield', to help prevent the formation of damaging protein aggregates during water stress.
Figures
Similar articles
-
MtPM25 is an atypical hydrophobic late embryogenesis-abundant protein that dissociates cold and desiccation-aggregated proteins.Plant Cell Environ. 2010 Mar;33(3):418-30. doi: 10.1111/j.1365-3040.2009.02093.x. Epub 2009 Nov 25. Plant Cell Environ. 2010. PMID: 20002332
-
Dehydration-specific induction of hydrophilic protein genes in the anhydrobiotic nematode Aphelenchus avenae.Eukaryot Cell. 2004 Aug;3(4):966-75. doi: 10.1128/EC.3.4.966-975.2004. Eukaryot Cell. 2004. PMID: 15302829 Free PMC article.
-
Identification of a novel LEA protein involved in freezing tolerance in wheat.Plant Cell Physiol. 2014 Jan;55(1):136-47. doi: 10.1093/pcp/pct164. Epub 2013 Nov 20. Plant Cell Physiol. 2014. PMID: 24265272
-
LEA proteins during water stress: not just for plants anymore.Annu Rev Physiol. 2011;73:115-34. doi: 10.1146/annurev-physiol-012110-142203. Annu Rev Physiol. 2011. PMID: 21034219 Review.
-
Physicochemical Aspects of the Biological Functions of Trehalose and Group 3 LEA Proteins as Desiccation Protectants.Adv Exp Med Biol. 2018;1081:271-286. doi: 10.1007/978-981-13-1244-1_15. Adv Exp Med Biol. 2018. PMID: 30288715 Review.
Cited by
-
QTLs and candidate genes for desiccation and abscisic acid content in maize kernels.BMC Plant Biol. 2010 Jan 4;10:2. doi: 10.1186/1471-2229-10-2. BMC Plant Biol. 2010. PMID: 20047666 Free PMC article.
-
Analysis of expressed sequence tags and identification of genes encoding cell-wall-degrading enzymes from the fungivorous nematode Aphelenchus avenae.BMC Genomics. 2009 Nov 16;10:525. doi: 10.1186/1471-2164-10-525. BMC Genomics. 2009. PMID: 19917084 Free PMC article.
-
Tardigrade small heat shock proteins can limit desiccation-induced protein aggregation.Commun Biol. 2023 Jan 30;6(1):121. doi: 10.1038/s42003-023-04512-y. Commun Biol. 2023. PMID: 36717706 Free PMC article.
-
Cryoprotective mechanism of a small intrinsically disordered dehydrin protein.Protein Sci. 2011 Jan;20(1):42-50. doi: 10.1002/pro.534. Protein Sci. 2011. PMID: 21031484 Free PMC article.
-
Integrated physiological and proteomic analysis of embryo and endosperm reveals central salt stress response proteins during seed germination of winter wheat cultivar Zhengmai 366.BMC Plant Biol. 2019 Jan 18;19(1):29. doi: 10.1186/s12870-019-1643-z. BMC Plant Biol. 2019. Retraction in: BMC Plant Biol. 2019 Sep 17;19(1):406. doi: 10.1186/s12870-019-2019-0. PMID: 30658564 Free PMC article. Retracted.
References
-
- Cuming A. C. LEA proteins. In: Shewry P. R., Casey R., editors. Seed Proteins. Dordrecht: Kluwer Academic Publishers; 1999. pp. 753–780.
-
- Welin B. V., Olson A., Nylander M., Palva E. T. Characterisation and differential expression of DHN/LEA/RAB-like genes during cold-acclimation and drought stress in Arabidopsis thaliana. Plant Mol. Biol. 1994;26:131–144. - PubMed
-
- Zhang L., Ohta A., Bray E. A., Imai R. Expression of plant group 2 and group 3 LEA proteins in Saccharomyces cerevisiae revealed functional divergence among LEA proteins. J. Biochem. (Tokyo) 2000;127:611–616. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

