Glycogen synthase kinase-3 is an endogenous inhibitor of Snail transcription: implications for the epithelial-mesenchymal transition
- PMID: 15631989
- PMCID: PMC2171685
- DOI: 10.1083/jcb.200409067
Glycogen synthase kinase-3 is an endogenous inhibitor of Snail transcription: implications for the epithelial-mesenchymal transition
Abstract
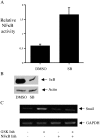
We report that the activity of glycogen synthase kinase-3 (GSK-3) is necessary for the maintenance of the epithelial architecture. Pharmacological inhibition of its activity or reducing its expression using small interfering RNAs in normal breast and skin epithelial cells results in a reduction of E-cadherin expression and a more mesenchymal morphology, both of which are features associated with an epithelial-mesenchymal transition (EMT). Importantly, GSK-3 inhibition also stimulates the transcription of Snail, a repressor of E-cadherin and an inducer of the EMT. We identify NFkappaB as a transcription factor inhibited by GSK-3 in epithelial cells that is relevant for Snail expression. These findings indicate that epithelial cells must sustain activation of a specific kinase to impede a mesenchymal transition.
Figures


References
-
- Barbera, M.J., I. Puig, D. Dominguez, S. Julien-Grille, S. Guaita-Esteruelas, S. Peiro, J. Baulida, C. Franci, S. Dedhar, L. Larue, and A. Garcia De Herreros. 2004. Regulation of Snail transcription during epithelial to mesenchymal transition of tumor cells. Oncogene. 23:7345–7354. - PubMed
-
- Batlle, E., E. Sancho, C. Franci, D. Dominguez, M. Monfar, J. Baulida, and A. Garcia De Herreros. 2000. The transcription factor snail is a repressor of E-cadherin gene expression in epithelial tumour cells. Nat. Cell Biol. 2:84–89. - PubMed
-
- Beals, C.R., C.M. Sheridan, C.W. Turck, P. Gardner, and G.R. Crabtree. 1997. Nuclear export of NF-ATc enhanced by glycogen synthase kinase-3. Science. 275:1930–1934. - PubMed
-
- Blanco, M.J., G. Moreno-Bueno, D. Sarrio, A. Locascio, A. Cano, J. Palacios, and M.A. Nieto. 2002. Correlation of Snail expression with histological grade and lymph node status in breast carcinomas. Oncogene. 21:3241–3246. - PubMed
-
- Bringuier, P.P., R. Umbas, H.E. Schaafsma, H.F. Karthaus, F.M. Debruyne, and J.A. Schalken. 1993. Decreased E-cadherin immunoreactivity correlates with poor survival in patients with bladder tumors. Cancer Res. 53:3241–3245. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

