Identification of cytoplasmic residues of Sec61p involved in ribosome binding and cotranslational translocation
- PMID: 15631991
- PMCID: PMC2171681
- DOI: 10.1083/jcb.200408188
Identification of cytoplasmic residues of Sec61p involved in ribosome binding and cotranslational translocation
Abstract
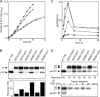
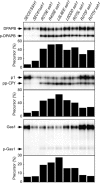
The cytoplasmic surface of Sec61p is the binding site for the ribosome and has been proposed to interact with the signal recognition particle receptor during targeting of the ribosome nascent chain complex to the translocation channel. Point mutations in cytoplasmic loops six (L6) and eight (L8) of yeast Sec61p cause reductions in growth rates and defects in the translocation of nascent polypeptides that use the cotranslational translocation pathway. Sec61 heterotrimers isolated from the L8 sec61 mutants have a greatly reduced affinity for 80S ribosomes. Cytoplasmic accumulation of protein precursors demonstrates that the initial contact between the large ribosomal subunit and the Sec61 complex is important for efficient insertion of a nascent polypeptide into the translocation pore. In contrast, point mutations in L6 of Sec61p inhibit cotranslational translocation without significantly reducing the ribosome-binding activity, indicating that the L6 and L8 sec61 mutants affect different steps in the cotranslational translocation pathway.
Figures





References
-
- Arnold, C.E., and K.D. Wittrup. 1994. The stress response to loss of signal recognition particle function in Saccharomyces cerevisiae. J. Biol. Chem. 269:30412–30418. - PubMed
-
- Beckmann, R., D. Bubeck, R. Grassucci, P. Penczek, A. Verschoor, G. Blobel, and J. Frank. 1997. Alignment of conduits for the nascent polypeptide chain in the ribosome-Sec61 complex. Science. 278:2123–2126. - PubMed
-
- Beckmann, R., C.M. Spahn, N. Eswar, J. Helmers, P.A. Penczek, A. Sali, J. Frank, and G. Blobel. 2001. Architecture of the protein-conducting channel associated with the translating 80S ribosome. Cell. 107:361–372. - PubMed
-
- Corsi, A.K., and R. Schekman. 1996. Mechanism of polypeptide translocation into the endoplasmic reticulum. J. Biol. Chem. 271:30299–30302. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

