Matrix metalloproteinase-9 knockout confers resistance to corneal epithelial barrier disruption in experimental dry eye
- PMID: 15632000
- PMCID: PMC1602302
- DOI: 10.1016/S0002-9440(10)62232-8
Matrix metalloproteinase-9 knockout confers resistance to corneal epithelial barrier disruption in experimental dry eye
Abstract
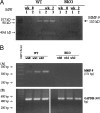
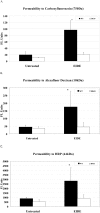
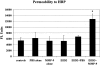
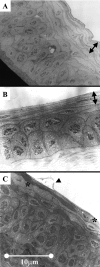
Altered corneal epithelial barrier function is the cause for ocular irritation and visual morbidity in dry eye disease. Increased matrix metalloproteinase (MMP)-9 activity has been observed in the tear fluid of dry eye patients. To determine the pathogenic role of MMP-9 in the corneal epithelial disease of dry eye, the effects of experimentally induced dry eye on corneal epithelial morphology and barrier function were compared in MMP-9 knockout mice and their wild-type littermates. Dry eye was created through cholinergic blockade and exposure to a desiccating environment. The tear fluid MMP-9 concentration increased in response to dryness in wild-type mice. Corneal epithelial permeability to three different-sized molecules increased in dry eye wild-type mice, but not in MMP-9 knockout mice. Topical administration of active MMP-9 to dry eye MMP-9 knockout mice significantly increased corneal epithelial permeability. Compared to MMP-9 knockout mice, wild-type mice showed greater desquamation of differentiated apical corneal epithelial cells that expressed the tight junction protein occludin in response to dryness. This was accompanied by an increase in lower sized (50 kd) occludin in the corneal epithelia of wild-type mice. These findings could be replicated in cultured human corneal epithelial cells that were treated with active MMP-9. These studies indicate that increased MMP-9 activity on the ocular surface in response to dryness disrupts corneal epithelial barrier function. This appears to be because of accelerated loss of tight junction bearing superficial corneal epithelial cells, perhaps by proteolytic cleavage of occludin.
Figures







References
-
- Murillo-Lopez F, Pflugfelder SC. Dry eye. Krachmer J, Mannis M, Holland E, editors. St. Louis: Mosby; The Cornea. 1996:pp 663–686.
-
- Yokoi N, Kinoshita S. Clinical evaluation of corneal epithelial barrier function with the slit-lamp fluorophotometer. Cornea. 1995;14:485–489. - PubMed
-
- Gobbels M, Spitznas M. Corneal epithelial permeability of dry eyes before and after treatment with artificial tears. Ophthalmology. 1992;99:873–878. - PubMed
-
- Pflugfelder SC, Tseng SC, Sanabria O, Kell H, Garcia CG, Felix C, Feuer W, Reis BL. Evaluation of subjective assessments and objective diagnostic tests for diagnosing tear-film disorders known to cause ocular irritation. Cornea. 1998;17:38–56. - PubMed
-
- Rolando M, Iester M, Macri A, Calabria G. Low spatial-contrast sensitivity in dry eyes. Cornea. 1998;17:376–379. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Miscellaneous

