Human herpesvirus 6 activates lytic cycle replication of Kaposi's sarcoma-associated herpesvirus
- PMID: 15632010
- PMCID: PMC1602294
- DOI: 10.1016/S0002-9440(10)62242-0
Human herpesvirus 6 activates lytic cycle replication of Kaposi's sarcoma-associated herpesvirus
Erratum in
-
Correction.Am J Pathol. 2021 May;191(5):965. doi: 10.1016/j.ajpath.2021.02.012. Am J Pathol. 2021. PMID: 33894887 Free PMC article. No abstract available.
Abstract
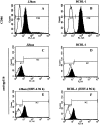
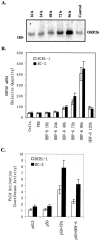
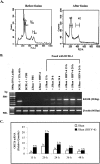
Kaposi's sarcoma-associated herpesvirus (KSHV) or human herpesvirus 8 (HHV-8) is a gamma-herpesvirus consistently identified in Kaposi's sarcoma (KS), primary effusion lymphoma, and multicentric Castleman's disease. KSHV infection appears to be necessary, but not be sufficient for development of KS without other co-factors. However, factors that facilitate KSHV to cause KS have not been well defined. Because patients with KS are often immunosuppressed and susceptible to many infectious agents including human herpesvirus 6 (HHV-6), we investigated the potential of HHV-6 to influence the replication of KSHV. By co-culturing HHV-6-infected T cells with KSHV-latent BCBL-1 cell line, infecting BCBL-1 cells with HHV-6 virions, and generating heterokaryons between HHV-6-infected T cells and BCBL-1 cells, we showed that HHV-6 played a critical role in induction of KSHV replication, as determined by production of lytic phase mRNA transcripts and viral proteins. We confirmed and extended the results by using a luciferase reporter assay in which KSHV ORF50 promoter, the first promoter activated during KSHV replication, drove the luciferase expression. Besides HHV-6, we also found that cytokines such as interferon-gamma partially contributed to induction of KSHV replication in the co-culture system. These findings suggest that HHV-6 may participate in KS pathogenesis by promoting KSHV replication and increasing KSHV viral load.
Figures
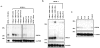


Similar articles
-
Intracellular Tat of human immunodeficiency virus type 1 activates lytic cycle replication of Kaposi's sarcoma-associated herpesvirus: role of JAK/STAT signaling.J Virol. 2007 Mar;81(5):2401-17. doi: 10.1128/JVI.02024-06. Epub 2006 Dec 6. J Virol. 2007. PMID: 17151125 Free PMC article.
-
Induction of HHV-8 lytic cycle replication by inflammatory cytokines produced by HIV-1-infected T cells.Am J Pathol. 2000 Jun;156(6):1961-71. doi: 10.1016/S0002-9440(10)65069-9. Am J Pathol. 2000. PMID: 10854219 Free PMC article.
-
Identification of Novel Kaposi's Sarcoma-Associated Herpesvirus Orf50 Transcripts: Discovery of New RTA Isoforms with Variable Transactivation Potential.J Virol. 2016 Dec 16;91(1):e01434-16. doi: 10.1128/JVI.01434-16. Print 2017 Jan 1. J Virol. 2016. PMID: 27795414 Free PMC article.
-
[Replication Machinery of Kaposi's Sarcoma-associated Herpesvirus and Drug Discovery Research].Yakugaku Zasshi. 2019;139(1):69-73. doi: 10.1248/yakushi.18-00164-2. Yakugaku Zasshi. 2019. PMID: 30606932 Review. Japanese.
-
Pathological Features of Kaposi's Sarcoma-Associated Herpesvirus Infection.Adv Exp Med Biol. 2018;1045:357-376. doi: 10.1007/978-981-10-7230-7_16. Adv Exp Med Biol. 2018. PMID: 29896675 Review.
Cited by
-
Activation of Kaposi's sarcoma-associated herpesvirus (KSHV) by inhibitors of class III histone deacetylases: identification of sirtuin 1 as a regulator of the KSHV life cycle.J Virol. 2014 Jun;88(11):6355-67. doi: 10.1128/JVI.00219-14. Epub 2014 Mar 26. J Virol. 2014. PMID: 24672028 Free PMC article.
-
Laboratory and clinical aspects of human herpesvirus 6 infections.Clin Microbiol Rev. 2015 Apr;28(2):313-35. doi: 10.1128/CMR.00122-14. Clin Microbiol Rev. 2015. PMID: 25762531 Free PMC article. Review.
-
Lymphoproliferative Syndromes Associated with Human Herpesvirus-6A and Human Herpesvirus-6B.Mediterr J Hematol Infect Dis. 2018 May 1;10(1):e2018035. doi: 10.4084/MJHID.2018.035. eCollection 2018. Mediterr J Hematol Infect Dis. 2018. PMID: 29755712 Free PMC article. Review.
-
Co-infections and Pathogenesis of KSHV-Associated Malignancies.Front Microbiol. 2016 Feb 15;7:151. doi: 10.3389/fmicb.2016.00151. eCollection 2016. Front Microbiol. 2016. PMID: 26913028 Free PMC article. Review.
-
Evidence of inability of human cytomegalovirus to reactivate Kaposi's sarcoma-associated herpesvirus from latency in body cavity-based lymphocytes.J Clin Virol. 2009 Nov;46(3):244-8. doi: 10.1016/j.jcv.2009.07.025. Epub 2009 Sep 1. J Clin Virol. 2009. PMID: 19726225 Free PMC article.
References
-
- Chang Y, Cesarman E, Pessin MS, Lee F, Culpepper J, Knowles DM, Moore PS. Identification of herpesvirus-like DNA sequences in AIDS-associated Kaposi’s sarcoma. Science. 1994;266:1865–1869. - PubMed
-
- Katano H, Sata T. Human herpesvirus 8: virology, epidemiology and related diseases. Jpn J Infect Dis. 2000;53:137–155. - PubMed
-
- Schulz TF. Kaposi’s sarcoma-associated herpesvirus (human herpesvirus-8). J Gen Virol. 1998;79:1573–1591. - PubMed
-
- Moore PS, Chang Y. Detection of herpesvirus-like DNA sequences in Kaposi’s sarcoma in patients with and without HIV infection. N Engl J Med. 1995;332:1181–1185. - PubMed
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Medical

