Cellular response of cardiac fibroblasts to amyloidogenic light chains
- PMID: 15632012
- PMCID: PMC1602293
- DOI: 10.1016/S0002-9440(10)62244-4
Cellular response of cardiac fibroblasts to amyloidogenic light chains
Abstract
Amyloidoses are a group of disorders characterized by abnormal folding of proteins that impair organ function. We investigated the cellular response of primary cardiac fibroblasts to amyloidogenic light chains and determined the corresponding change in proteoglycan expression and localization. The cellular response to 11 urinary immunoglobulin light chains of kappa1, lambda6, and lambda 3 subtypes was evaluated. The localization of the light chains was monitored by conjugating them to Oregon Green 488 and performing live cell confocal microscopy. Sulfation of the proteoglycans was determined after elution over Q1-columns with a single-step salt gradient (1.5 mol/L NaCl) via dimethylmethylene blue. Light chains were detected inside cells within 4 hours and demonstrated perinuclear localization. Over 80% of the cells showed intracellular localization of the amyloid light chains. The light chains induced sulfation of the secreted glycosaminoglycans, but the cell fraction possessed only minimal sulfation. Furthermore, the light chains caused a translocation of heparan sulfate proteoglycan to the nucleus. The conformation and thermal stability of light chains was altered when they were incubated in the presence of heparan sulfate and destabilization of the amyloid light chains was detected. These studies indicate that internalization of the light chains mediates the expression and localization of heparan sulfate proteoglycans.
Figures


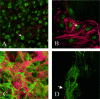
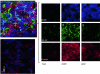



Similar articles
-
Role of endocytic inhibitory drugs on internalization of amyloidogenic light chains by cardiac fibroblasts.Am J Pathol. 2006 Dec;169(6):1939-52. doi: 10.2353/ajpath.2006.060183. Am J Pathol. 2006. PMID: 17148659 Free PMC article.
-
Immunoglobulin light chains, glycosaminoglycans, and amyloid.Cell Mol Life Sci. 2000 Mar;57(3):441-9. doi: 10.1007/PL00000706. Cell Mol Life Sci. 2000. PMID: 10823245 Free PMC article. Review.
-
Role of glycosaminoglycan sulfation in the formation of immunoglobulin light chain amyloid oligomers and fibrils.J Biol Chem. 2010 Nov 26;285(48):37672-82. doi: 10.1074/jbc.M110.149575. Epub 2010 Sep 24. J Biol Chem. 2010. PMID: 20870723 Free PMC article.
-
Identification of amyloidogenic light chains requires the combination of serum-free light chain assay with immunofixation of serum and urine.Clin Chem. 2009 Mar;55(3):499-504. doi: 10.1373/clinchem.2008.117143. Epub 2009 Jan 8. Clin Chem. 2009. PMID: 19131635
-
Review: immunoglobulin light chain amyloidosis--the archetype of structural and pathogenic variability.J Struct Biol. 2000 Jun;130(2-3):280-9. doi: 10.1006/jsbi.2000.4248. J Struct Biol. 2000. PMID: 10940232 Review.
Cited by
-
Immunoglobulin light chain amyloid aggregation.Chem Commun (Camb). 2018 Sep 20;54(76):10664-10674. doi: 10.1039/c8cc04396e. Chem Commun (Camb). 2018. PMID: 30087961 Free PMC article. Review.
-
Understanding AL amyloidosis with a little help from in vivo models.Front Immunol. 2022 Nov 15;13:1008449. doi: 10.3389/fimmu.2022.1008449. eCollection 2022. Front Immunol. 2022. PMID: 36458006 Free PMC article. Review.
-
Role of endocytic inhibitory drugs on internalization of amyloidogenic light chains by cardiac fibroblasts.Am J Pathol. 2006 Dec;169(6):1939-52. doi: 10.2353/ajpath.2006.060183. Am J Pathol. 2006. PMID: 17148659 Free PMC article.
-
Cytotoxicity of amyloidogenic immunoglobulin light chains in cell culture.Cell Death Dis. 2010 Nov 11;1(11):e98. doi: 10.1038/cddis.2010.75. Cell Death Dis. 2010. PMID: 21368874 Free PMC article.
-
Cardiac Troponin in Patients With Light Chain and Transthyretin Cardiac Amyloidosis: JACC: CardioOncology State-of-the-Art Review.JACC CardioOncol. 2024 Feb 20;6(1):1-15. doi: 10.1016/j.jaccao.2023.12.006. eCollection 2024 Feb. JACC CardioOncol. 2024. PMID: 38510286 Free PMC article. Review.
References
-
- Merlini G, Bellotti V. Molecular mechanisms of amyloidosis. N Engl J Med. 2003;349:583–596. - PubMed
-
- Kyle RA, Gertz MA. Primary systemic amyloidosis: clinical and laboratory features in 474 cases. Semin Hematol. 1995;32:45–59. - PubMed
-
- Pascali E. Diagnosis and treatment of primary amyloidosis. Crit Rev Oncol Hematol. 1995;19:149–181. - PubMed
-
- Brenner DA, Jain M, Pimentel DR, Wang B, Connors LH, Skinner M, Apstein CS, Liao R. Human amyloidogenic light chains directly impair cardiomyocyte function through an increase in cellular oxidant stress. Circ Res. 2004;94:1008–1010. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

