Melanocyte-specific proteins are aberrantly trafficked in melanocytes of Hermansky-Pudlak syndrome-type 3
- PMID: 15632015
- PMCID: PMC1602298
- DOI: 10.1016/S0002-9440(10)62247-X
Melanocyte-specific proteins are aberrantly trafficked in melanocytes of Hermansky-Pudlak syndrome-type 3
Abstract
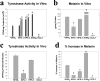
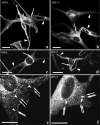
Hermansky-Pudlak Syndrome-type 3 (HPS-3) is a relatively mild subtype of HPS with minimal cutaneous and ocular depigmentation. The HPS-3 gene encodes a novel protein of unknown function with a predicted molecular weight of 114 kd. To assess the role of the HPS3 protein in melanization, cultured melanocytes developed from HPS-3 patients were evaluated biochemically and histologically for activity and localization of melanocyte-specific proteins. Endogenous tyrosinase activity of HPS-3 melanocytes was substantial, but tyrosinase activity and melanin synthesis was suppressed in intact melanocytes. However, the level of suppression, as well as extent to which up-regulation by isobutylmethylxanthine and cholera toxin was muted, was less that in HPS-1 melanocytes. Ultrastructurally, HPS-3 melanocytes contained morphologically normal melanosomes, predominantly of stage I and II with minimal stage III and few stage IV melanosomes. Dihydroxyphenylalanine (DOPA) histochemistry demonstrated an increase in melanization of melanosomes. Unique to HPS-3 melanocytes were numerous DOPA-positive 50-nm vesicles and tubular elements present throughout the cell body and dendrites. Tyrosinase, tyrosinase-related protein-1 (Tyrp1), dopachrome tautomerase (Dct), and LAMP1 and 3 localization in HPS-3 melanocytes, as evaluated by immunocytochemistry and confocal microscopy, demonstrated a fine, floccular distribution in contrast to the coarse, granular distribution characteristic of control melanocytes. The localization profile of other proteins expressed by melanocytes (ie, Silver/Pmel17, Melan-A/MART-1, LAMP2, Rab 27, transferrin, c-kit, adaptin-3, and the HPS1 protein) appeared normal. These results suggest that a specific subset of melanocyte proteins are aberrantly trafficked throughout the HPS-3 melanocyte and may be responsible for the reduction in melanin synthesis.
Figures




References
-
- Hermansky F, Pudlak P. Albinism associated with hemorrhagic diathesis and unusual pigmented reticular cells in the bone marrow: report of two cases with histochemical studies. Blood. 1959;14:162–169. - PubMed
-
- Huizing M, Boissy RE, Gahl WA. Hermansky-Pudlak syndrome: vesicle formation from yeast to man. Pigment Cell Res. 2002;15:405–419. - PubMed
-
- King RA, Hearing VJ, Creel DJ, Oetting WS. Albinism. Scriver CR, Beaudet AL, Valle DL, Sly WS, editors. New York: McGraw-Hill; The Metabolic and Molecular Bases of Inherited Disease. 2001:pp 5587–5627.
-
- Witkop CJJ, Krumwiede M, Sedano H, White JG. Reliability of absent platelet dense bodies as a diagnostic criterion for Hermansky-Pudlak syndrome. Am J Hematol. 1987;26:305–311. - PubMed
-
- Gahl WA, Brantly M, Kaiser-Kupfer MI, Iwata F, Hazelwood S, Shotelersuk V, Duffy LF, Kuehl EM, Troendle I, Bernardini I. Genetic defects and clinical characteristics of patients with a form of oculocutaneous albinism (Hermansky-Pudlak syndrome). N Engl J Med. 1998;338:1258–1264. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

