Brain and bone damage in KARAP/DAP12 loss-of-function mice correlate with alterations in microglia and osteoclast lineages
- PMID: 15632019
- PMCID: PMC1602283
- DOI: 10.1016/S0002-9440(10)62251-1
Brain and bone damage in KARAP/DAP12 loss-of-function mice correlate with alterations in microglia and osteoclast lineages
Abstract
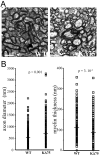
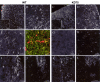
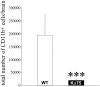
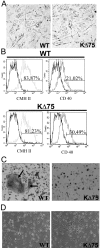
Human polycystic lipomembraneous osteodysplasia with sclerosing leukoencephalopathy, also known as Nasu-Hakola disease, has been described to be associated with mutations affecting the immunoreceptor tyrosine-based activation motif-bearing KARAP/DAP12 immunoreceptor gene. Patients present bone fragilities and severe neurological alterations leading to presenile dementia. Here we investigated whether the absence of KARAP/DAP12-mediated signals in loss-of-function (KDelta75) mice also leads to bone and central nervous system pathological features. Histological analysis of adult KDelta75 mice brains revealed a diffuse hypomyelination predominating in anterior brain regions. As this was not accompanied by oligodendrocyte degeneration or microglial cell activation it suggests a developmental defect of myelin formation. Interestingly, in postnatal KDelta75 mice, we observed a dramatic reduction in microglial cell numbers similar to in vitro microglial cell differentiation impairment. Our results raise the intriguing possibility that defective microglial cell differentiation might be responsible for abnormal myelin development. Histomorphometry revealed that bone remodeling is also altered, because of a resorption defect, associated with a severe block of in vitro osteoclast differentiation. In addition, we show that, among monocytic lineages, KARAP/DAP12 specifically controls microglial and osteoclast differentiation. Our results confirm that KARAP/DAP12-mediated signals play an important role in the regulation of both brain and bone homeostasis. Yet, important differences exist between the symptoms observed in Nasu-Hakola patients and KDelta75 mice.
Figures




References
-
- Olcese L, Cambiaggi A, Semenzato G, Bottino C, Moretta A, Vivier E. Human killer cell activatory receptors for MHC class I molecules are included in a multimeric complex expressed by natural killer cells. J Immunol. 1997;158:5083–5086. - PubMed
-
- Lanier LL, Corliss BC, Wu J, Leong C, Phillips JH. Immunoreceptor DAP12 bearing a tyrosine-based activation motif is involved in activating NK cells. Nature. 1998;391:703–707. - PubMed
-
- Tomasello E, Blery M, Vely F, Vivier E. Signaling pathways engaged by NK cell receptors: double concerto for activating receptors, inhibitory receptors and NK cells. Semin Immunol. 2000;12:139–147. - PubMed
-
- Moretta A, Bottino C, Vitale M, Pende D, Cantoni C, Mingari MC, Biassoni R, Moretta L. Activating receptors and coreceptors involved in human natural killer cell-mediated cytolysis. Annu Rev Immunol. 2001;19:197–223. - PubMed
-
- Diefenbach A, Tomasello E, Lucas M, Jamieson AM, Hsia JK, Vivier E, Raulet DH. Selective associations with signaling proteins determine stimulatory versus costimulatory activity of NKG2D. Nat Immunol. 2002;3:1142–1149. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Research Materials

