Administration of ricin induces a severe inflammatory response via nonredundant stimulation of ERK, JNK, and P38 MAPK and provides a mouse model of hemolytic uremic syndrome
- PMID: 15632024
- PMCID: PMC1602309
- DOI: 10.1016/S0002-9440(10)62256-0
Administration of ricin induces a severe inflammatory response via nonredundant stimulation of ERK, JNK, and P38 MAPK and provides a mouse model of hemolytic uremic syndrome
Abstract
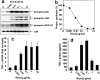
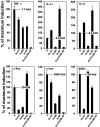
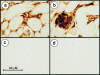
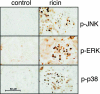
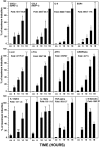
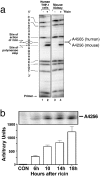
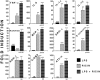
Recent interest in the health consequences of ricin as a weapon of terrorism has led us to investigate the effects of ricin on cells in vitro and in mice. Our previous studies showed that depurination of the 28S rRNA by ricin results in the inhibition of translation and the coordinate activation of the stress-activated protein kinases JNK and p38 MAPK. In RAW 264.7 macrophages, ricin induced the activation of ERK, JNK, and p38 MAPK, the accumulation of mRNA encoding tumor necrosis factor (TNF)-alpha, interleukin (IL)-1, the transcription factors c-Fos, c-Jun, and EGR1, and the appearance of TNF-alpha protein in the culture medium. Using specific inhibitors of MAPKs, we demonstrated the nonredundant roles of the individual MAPKs in mediating proinflammatory gene activation in response to ricin. Similarly, the intravenous administration of ricin to mice led to the activation of ERK, JNK, and p38 MAPK in the kidneys, and increases in plasma-borne TNF-alpha, IL-1beta, and IL-6. Ricin-injected mice developed the hallmarks of hemolytic uremic syndrome, including thrombotic microangiopathy, hemolytic anemia, thrombocytopenia, and acute renal failure. Microarray analyses demonstrated a massive proinflammatory transcriptional response in the kidneys, coincidental with the symptoms of hemolytic uremic syndrome. Therapeutic management of the inflammatory response may affect the outcome of intoxication by ricin.
Figures


Similar articles
-
HMG-CoA reductase inhibitors upregulate heme oxygenase-1 expression in murine RAW264.7 macrophages via ERK, p38 MAPK and protein kinase G pathways.Cell Signal. 2006 Jan;18(1):32-9. doi: 10.1016/j.cellsig.2005.03.016. Epub 2005 Apr 20. Cell Signal. 2006. PMID: 16214041
-
Ribotoxic stress response to the trichothecene deoxynivalenol in the macrophage involves the SRC family kinase Hck.Toxicol Sci. 2005 Jun;85(2):916-26. doi: 10.1093/toxsci/kfi146. Epub 2005 Mar 16. Toxicol Sci. 2005. PMID: 15772366
-
NF-κB, ERK, p38 MAPK and JNK contribute to the initiation and/or maintenance of mechanical allodynia induced by tumor necrosis factor-alpha in the red nucleus.Brain Res Bull. 2013 Oct;99:132-9. doi: 10.1016/j.brainresbull.2013.10.008. Epub 2013 Oct 23. Brain Res Bull. 2013. PMID: 24161765
-
p38 pathway kinases as anti-inflammatory drug targets.J Dent Res. 2007 Sep;86(9):800-11. doi: 10.1177/154405910708600902. J Dent Res. 2007. PMID: 17720847 Review.
-
MAP kinase and pain.Brain Res Rev. 2009 Apr;60(1):135-48. doi: 10.1016/j.brainresrev.2008.12.011. Epub 2008 Dec 25. Brain Res Rev. 2009. PMID: 19150373 Free PMC article. Review.
Cited by
-
Intracellular Transport and Cytotoxicity of the Protein Toxin Ricin.Toxins (Basel). 2019 Jun 18;11(6):350. doi: 10.3390/toxins11060350. Toxins (Basel). 2019. PMID: 31216687 Free PMC article. Review.
-
Oridonin suppresses gastric cancer SGC-7901 cell proliferation by targeting the TNF-alpha/androgen receptor/TGF-beta signalling pathway axis.J Cell Mol Med. 2023 Sep;27(18):2661-2674. doi: 10.1111/jcmm.17841. Epub 2023 Jul 11. J Cell Mol Med. 2023. PMID: 37431884 Free PMC article.
-
Pokeweed antiviral protein increases HIV-1 particle infectivity by activating the cellular mitogen activated protein kinase pathway.PLoS One. 2012;7(5):e36369. doi: 10.1371/journal.pone.0036369. Epub 2012 May 1. PLoS One. 2012. PMID: 22563495 Free PMC article.
-
Plant ribosome-inactivating proteins type II induce the unfolded protein response in human cancer cells.Cell Mol Life Sci. 2011 Apr;68(7):1269-81. doi: 10.1007/s00018-010-0524-2. Epub 2010 Sep 16. Cell Mol Life Sci. 2011. PMID: 20844919 Free PMC article.
-
Deoxynivalenol induces p38 interaction with the ribosome in monocytes and macrophages.Toxicol Sci. 2008 Sep;105(1):59-66. doi: 10.1093/toxsci/kfn102. Epub 2008 May 22. Toxicol Sci. 2008. PMID: 18502741 Free PMC article.
References
-
- Franz DR, Jaax JK. Ricin toxin. Sidell FR, Takfuji ET, Franz DR, editors. Washington: Office of the Surgeon General, Department of the Army,; Textbook of Military Medicine, Part 1. Warfare, Weaponry, and the Casualty: Medical Aspects of Chemical and Biological Warfare. 1997:pp 631–642.
-
- Smallshaw JE, Firan A, Fulmer JR, Ruback SL, Ghetie V, Vitetta ES. A novel recombinant vaccine which protects mice against ricin intoxication. Vaccine. 2002;20:3422–3427. - PubMed
-
- Crompton R, Gall D. Georgi Markov—death in a pellet. Med Leg J. 1980;48:51–62. - PubMed
-
- Barbieri L, Battelli MG, Stirpe F. Ribosome-inactivating proteins from plants. Biochim Biophys Acta. 1993;1154:237–282. - PubMed
-
- Iordanov MS, Pribnow D, Magun JL, Dinh TH, Pearson JA, Chen SL, Magun BE. Ribotoxic stress response: activation of the stress-activated protein kinase JNK1 by inhibitors of the peptidyl transferase reaction and by sequence-specific RNA damage to the alpha-sarcin/ricin loop in the 28S rRNA. Mol Cell Biol. 1997;17:3373–3381. - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

