Receptor salvage from the prevacuolar compartment is essential for efficient vacuolar protein targeting
- PMID: 15632053
- PMCID: PMC544495
- DOI: 10.1105/tpc.104.026351
Receptor salvage from the prevacuolar compartment is essential for efficient vacuolar protein targeting
Abstract
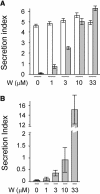
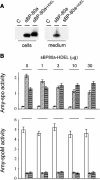
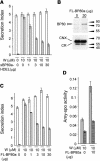
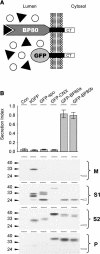
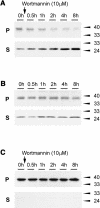
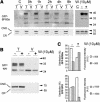
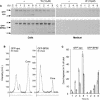
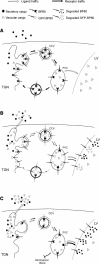
We have characterized the requirements to inhibit the function of the plant vacuolar sorting receptor BP80 in vivo and gained insight into the crucial role of receptor recycling between the prevacuolar compartment and the Golgi apparatus. The drug wortmannin interferes with the BP80-mediated route to the vacuole and induces hypersecretion of a soluble BP80-ligand. Wortmannin does not prevent receptor-ligand binding itself but causes BP80 levels to be limiting. Consequently, overexpression of BP80 partially restores vacuolar cargo transport. To simulate receptor traffic, we tested a truncated BP80 derivative in which the entire lumenal domain of BP80 has been replaced by the green fluorescent protein (GFP). The resulting chimeric protein (GFP-BP80) accumulates in the prevacuolar compartment as expected, but a soluble GFP fragment can also be detected in purified vacuoles. Interestingly, GFP-BP80 coexpression interferes with the correct sorting of a BP80-ligand and causes hypersecretion that is reversible by expressing a 10-fold excess of full-length BP80. This suggests that GFP-BP80 competes with endogenous BP80 mainly at the retrograde transport route that rescues receptors from the prevacuolar compartment. Treatment with wortmannin causes further leakage of GFP-BP80 from the prevacuolar compartment to the vacuoles, whereas BP80-ligands are secreted. We propose that recycling of the vacuolar sorting receptor from the prevacuolar compartment to the Golgi apparatus is an essential process that is saturable and wortmannin sensitive.
Figures


References
-
- Ahmed, S.U., Rojo, E., Kovaleva, V., Venkataraman, S., Dombrowski, J.E., Matsuoka, K., and Raikhel, N.V. (2000). The plant vacuolar sorting receptor AtELP is involved in transport of NH(2)-terminal propeptide-containing vacuolar proteins in Arabidopsis thaliana. J. Cell Biol. 149, 1335–1344. - PMC - PubMed
-
- Bassham, D.C., and Raikhel, N.V. (2000). Unique features of the plant vacuolar sorting machinery. Curr. Opin. Cell Biol. 12, 491–495. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

