Phosphatidylinositol 3-kinase-mediated effects of glucose on vacuolar H+-ATPase assembly, translocation, and acidification of intracellular compartments in renal epithelial cells
- PMID: 15632060
- PMCID: PMC543406
- DOI: 10.1128/MCB.25.2.575-589.2005
Phosphatidylinositol 3-kinase-mediated effects of glucose on vacuolar H+-ATPase assembly, translocation, and acidification of intracellular compartments in renal epithelial cells
Abstract
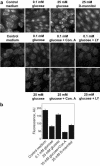
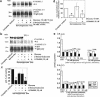
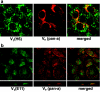
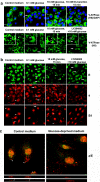
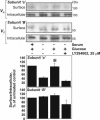
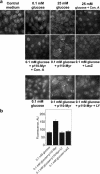
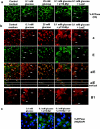
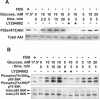
Vacuolar H+-ATPases (V-ATPases) are a family of ATP-driven proton pumps. They maintain pH gradients between intracellular compartments and are required for proton secretion out of the cytoplasm. Mechanisms of extrinsic control of V-ATPase are poorly understood. Previous studies showed that glucose is an important regulator of V-ATPase assembly in Saccharomyces cerevisiae. Human V-ATPase directly interacts with aldolase, providing a coupling mechanism for glucose metabolism and V-ATPase function. Here we show that glucose is a crucial regulator of V-ATPase in renal epithelial cells and that the effect of glucose is mediated by phosphatidylinositol 3-kinase (PI3K). Glucose stimulates V-ATPase-dependent acidification of the intracellular compartments in human proximal tubular cells HK-2 and porcine renal epithelial cells LLC-PK1. Glucose induces rapid ATP-independent assembly of the V1 and Vo domains of V-ATPase and extensive translocation of the V-ATPase V1 and Vo domains between different membrane pools and between membranes and the cytoplasm. In HK-2 cells, glucose stimulates polarized translocation of V-ATPase to the apical plasma membrane. The effects of glucose on V-ATPase trafficking and assembly can be abolished by pretreatment with the PI3K inhibitor LY294002 and can be reproduced in glucose-deprived cells by adenoviral expression of the constitutively active catalytic subunit p110alpha of PI3K. Taken together these data provide evidence that, in renal epithelial cells, glucose plays an important role in the control of V-ATPase-dependent acidification of intracellular compartments and V-ATPase assembly and trafficking and that the effects of glucose are mediated by PI3K-dependent signaling.
Figures


References
-
- Belham, C., S. Wu, and J. Avruch. 1999. Intracellular signalling: PDK1-a kinase at the hub of things. Curr. Biol. 9:R93-R96. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
