The parafibromin tumor suppressor protein is part of a human Paf1 complex
- PMID: 15632063
- PMCID: PMC543415
- DOI: 10.1128/MCB.25.2.612-620.2005
The parafibromin tumor suppressor protein is part of a human Paf1 complex
Abstract
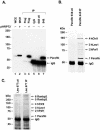
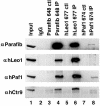
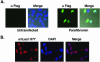
Parafibromin, the product of the HRPT2 (hyperparathyroidism-jaw tumor syndrome 2) tumor suppressor gene, is the human homologue of yeast Cdc73, part of the yeast RNA polymerase II/Paf1 complex known to be important for histone modification and connections to posttranscriptional events. By purifying cellular parafibromin and characterizing its associated proteins, we have identified a human counterpart to the yeast Paf1 complex including homologs of Leo1, Paf1, and Ctr9. Like the yeast complex, the parafibromin complex associates with the nonphosphorylated and Ser2 and Ser5 phosphorylated forms of the RNA polymerase II large subunit. Immunofluorescence experiments show that parafibromin is a nuclear protein. In addition, cotransfection data suggest that parafibromin can interact with a histone methyltransferase complex that methylates histone H3 on lysine 4. Some mutant forms of parafibromin lack association with hPaf1 complex members and with the histone methyltransferase complex, suggesting that disruption of these complexes may correlate with the oncogenic process.
Figures




References
-
- Betz, J. L., M. Chang, T. M. Washburn, S. E. Porter, C. L. Mueller, and J. A. Jaehning. 2002. Phenotypic analysis of Paf1/RNA polymerase II complex mutations reveals connections to cell cycle regulation, protein synthesis, and lipid and nucleic acid metabolism. Mol. Genet. Genomics 268:272-285. - PubMed
-
- Carpten, J. D., C. M. Robbins, A. Villablanca, L. Forsberg, S. Presciuttini, J. Bailey-Wilson, W. F. Simonds, E. M. Gillanders, A. M. Kennedy, J. D. Chen, S. K. Agarwal, R. Sood, M. P. Jones, T. Y. Moses, C. Haven, D. Petillo, P. D. Leotlela, B. Harding, D. Cameron, A. A. Pannett, A. Hoog, H. Heath III, L. A. James-Newton, B. Robinson, R. J. Zarbo, B. M. Cavaco, W. Wassif, N. D. Perrier, I. B. Rosen, U. Kristoffersson, P. D. Turnpenny, L. O. Farnebo, G. M. Besser, C. E. Jackson, H. Morreau, J. M. Trent, R. V. Thakker, S. J. Marx, B. T. Teh, C. Larsson, and M. R. Hobbs. 2002. HRPT2, encoding parafibromin, is mutated in hyperparathyroidism-jaw tumor syndrome. Nat. Genet. 32:676-680. - PubMed
-
- Gerber, M., and A. Shilatifard. 2003. Transcriptional elongation by RNA polymerase II and histone methylation. J. Biol. Chem. 278:26303-26306. - PubMed
-
- Goo, Y. H., Y. C. Sohn, D. H. Kim, S. W. Kim, M. J. Kang, D. J. Jung, E. Kwak, N. A. Barlev, S. L. Berger, V. T. Chow, R. G. Roeder, D. O. Azorsa, P. S. Meltzer, P. G. Suh, E. J. Song, K. J. Lee, Y. C. Lee, and J. W. Lee. 2003. Activating signal cointegrator 2 belongs to a novel steady-state complex that contains a subset of trithorax group proteins. Mol. Cell. Biol. 23:140-149. - PMC - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials
