Tenomodulin is necessary for tenocyte proliferation and tendon maturation
- PMID: 15632070
- PMCID: PMC543433
- DOI: 10.1128/MCB.25.2.699-705.2005
Tenomodulin is necessary for tenocyte proliferation and tendon maturation
Abstract
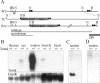
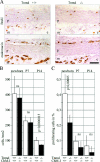
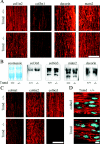
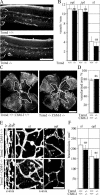
Tenomodulin (Tnmd) is a member of a new family of type II transmembrane glycoproteins. It is predominantly expressed in tendons, ligaments, and eyes, whereas the only other family member, chondromodulin I (ChM-I), is highly expressed in cartilage and at lower levels in the eye and thymus. The C-terminal extracellular domains of both proteins were shown to modulate endothelial-cell proliferation and tube formation in vitro and in vivo. We analyzed Tnmd function in vivo and provide evidence that Tnmd is processed in vivo and that the proteolytically cleaved C-terminal domain can be found in tendon extracts. Loss of Tnmd expression in gene targeted mice abated tenocyte proliferation and led to a reduced tenocyte density. The deposited amounts of extracellular matrix proteins, including collagen types I, II, III, and VI and decorin, lumican, aggrecan, and matrilin-2, were not affected, but the calibers of collagen fibrils varied significantly and exhibited increased maximal diameters. Tnmd-deficient mice did not have changes in tendon vessel density, and mice lacking both Tnmd and ChM-I had normal retinal vascularization and neovascularization after oxygen-induced retinopathy. These results suggest that Tnmd is a regulator of tenocyte proliferation and is involved in collagen fibril maturation but do not confirm an in vivo involvement of Tnmd in angiogenesis.
Figures

References
-
- Alon, T., I. Hemo, A. Itin, J. Pe'er, J. Stone, and E. Keshet. 1995. Vascular endothelial growth factor acts as a survival factor for newly formed retinal vessels and has implications for retinopathy of prematurity. Nat. Med. 1:1024-1028. - PubMed
-
- Azizan, A., N. Holaday, and P. J. Neame. 2001. Post-translational processing of bovine chondromodulin-I. J. Biol. Chem. 276:23632-23638. - PubMed
-
- Barr, P. J. 1991. Mammalian subtilisins: the long-sought dibasic processing endoproteases. Cell 66:1-3. - PubMed
-
- Benjamin, M., and J. R. Ralphs. 2000. The cell and developmental biology of tendons and ligaments. Int. Rev. Cytol. 196:85-130. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Miscellaneous
