Effects of oxymatrine on experimental hepatic fibrosis and its mechanism in vivo
- PMID: 15633229
- PMCID: PMC4205415
- DOI: 10.3748/wjg.v11.i2.268
Effects of oxymatrine on experimental hepatic fibrosis and its mechanism in vivo
Abstract
Aim: Hepatic fibrogenesis has close relation with hepatic stellate cells (HSC) and tissue inhibitors of metalloproteinase (TIMP). Oxymatrine (OM) is a kind of Chinese herb that is found to have some effects on liver fibrosis. We aimed to determine the effects of OM on hepatic fibrosis and explore the possible mechanism.
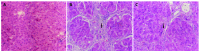
Methods: Thirty-two rats were randomly divided into four groups; 16 were used to develop hepatic fibrosis by carbon tetrachloride (CCl4) and treated with or without OM, and 16 were used as controls. The expression of tissue inhibitor of metalloproteinase-1 (TIMP-1) and alpha-smooth muscle actin (alpha-SMA) in the livers of rats was detected by immunohistochemical assay. Liver pathology was determined by H and E staining and reticulum staining.
Results: In CCl4-injured rats, the normal structure of lobules was destroyed, and pseudolobules were formed. Hyperplasia of fibers was observed surrounding the lobules. While the degree of fibrogenesis in liver tissues was significantly decreased in those rats with OM-treatment compared with those without OM treatment. The pseudolobules were surrounded by strong, multi-layer reticular fibers, which netted into pseudolobules in CCl4-injured rats, however, there was a significant decrease in reticular fibers in OM-treated rats. The expression of TIMP-1 in hepatic cells was weak in control groups, but strong in CCl4-injured groups, however, the expression of TIMP-1 was significantly inhibited by OM (F = 52.93, P<0.05). There was no significant change in the expression of alpha-SMA between CCl4-injured rats with or without OM treatment (F = 8.99, P>0.05).
Conclusion: OM effectively inhibits CCl4-induced fibrogenesis in rat liver tissues, probably by reducing the expression level of TIMP-1.
Figures




Similar articles
-
Oxymatrine liposome attenuates hepatic fibrosis via targeting hepatic stellate cells.World J Gastroenterol. 2012 Aug 21;18(31):4199-206. doi: 10.3748/wjg.v18.i31.4199. World J Gastroenterol. 2012. PMID: 22919254 Free PMC article.
-
[Effects of Shaoqiduogan on MMP-13, TIMP-1 expression in liver and hepatic stellate cells of hepatic fibrosis rats].Zhongguo Zhong Yao Za Zhi. 2010 Jun;35(11):1447-51. Zhongguo Zhong Yao Za Zhi. 2010. PMID: 20822018 Chinese.
-
[Inhibitory effect of acupuncture on hepatic extracellular matrix production in carbon tetrachloride-induced liver fibrosis rats].Zhen Ci Yan Jiu. 2012 Feb;37(1):8-14. Zhen Ci Yan Jiu. 2012. PMID: 22574562 Chinese.
-
Pay attention to the study on active antiliver fibrosis components of Chinese herbal medicine.Chin J Integr Med. 2012 Aug;18(8):563-4. doi: 10.1007/s11655-012-1029-7. Epub 2012 Mar 21. Chin J Integr Med. 2012. PMID: 22438172 Review.
-
[Forefront of therapy for hepatic fibrosis].Nihon Shokakibyo Gakkai Zasshi. 2002 Apr;99(4):365-78. Nihon Shokakibyo Gakkai Zasshi. 2002. PMID: 11979734 Review. Japanese. No abstract available.
Cited by
-
Oxymatrine liposome attenuates hepatic fibrosis via targeting hepatic stellate cells.World J Gastroenterol. 2012 Aug 21;18(31):4199-206. doi: 10.3748/wjg.v18.i31.4199. World J Gastroenterol. 2012. PMID: 22919254 Free PMC article.
-
Targeting Hepatic Stellate Cells for the Treatment of Liver Fibrosis by Natural Products: Is It the Dawning of a New Era?Front Pharmacol. 2020 Apr 30;11:548. doi: 10.3389/fphar.2020.00548. eCollection 2020. Front Pharmacol. 2020. PMID: 32425789 Free PMC article. Review.
-
Specific siRNA targeting the receptor for advanced glycation end products inhibits experimental hepatic fibrosis in rats.Int J Mol Sci. 2008 Apr;9(4):638-661. doi: 10.3390/ijms9040638. Epub 2008 Apr 24. Int J Mol Sci. 2008. PMID: 19325776 Free PMC article.
-
Understanding the Potential Role of Nanotechnology in Liver Fibrosis: A Paradigm in Therapeutics.Molecules. 2023 Mar 20;28(6):2811. doi: 10.3390/molecules28062811. Molecules. 2023. PMID: 36985782 Free PMC article. Review.
-
Defining the mechanisms behind the hepatoprotective properties of curcumin.Arch Toxicol. 2024 Aug;98(8):2331-2351. doi: 10.1007/s00204-024-03758-7. Epub 2024 Jun 5. Arch Toxicol. 2024. PMID: 38837048 Review.
References
-
- Friedman SL. Molecular regulation of hepatic fibrosis, an integrated cellular response to tissue injury. J Biol Chem. 2000;275:2247–2250. - PubMed
-
- Dodig M, Mullen KD. New mechanism of selective killing of activated hepatic stellate cells. Hepatology. 2003;38:1051–1053. - PubMed
-
- Cai Y, Shen XZ, Wang JY. Effects of glycyrrhizin on genes expression during the process of liver fibrosis. Zhonghua YiXue ZaZhi. 2003;83:1122–1125. - PubMed
-
- Nishio A, Keeffe EB, Gershwin ME. Immunopathogenesis of primary biliary cirrhosis. Semin Liver Dis. 2002;22:291–302. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

