Protons trap NR1/NR2B NMDA receptors in a nonconducting state
- PMID: 15634765
- PMCID: PMC6725198
- DOI: 10.1523/JNEUROSCI.3154-04.2005
Protons trap NR1/NR2B NMDA receptors in a nonconducting state
Abstract
NMDA receptors are highly expressed in the CNS and are involved in excitatory synaptic transmission, as well as synaptic plasticity. Given that overstimulation of NMDA receptors can cause cell death, it is not surprising that these channels are under tight control by a series of inhibitory extracellular ions, including zinc, magnesium, and H+. We studied the inhibition by extracellular protons of recombinant NMDA receptor NR1/NR2B single-channel and macroscopic responses in transiently transfected human embryonic kidney HEK 293 cells using patch-clamp techniques. We report that proton inhibition proceeds identically in the absence or presence of agonist, which rules out the possibility that protonation inhibits receptors by altering coagonist binding. The response of macroscopic currents in excised patches to rapid jumps in pH was used to estimate the microscopic association and dissociation rates for protons, which were 1.4 x 10(9) m(-1) sec(-1) and 110-196 sec(-1), respectively (K(d) corresponds to pH 7.2). Protons reduce the open probability without altering the time course of desensitization or deactivation. Protons appear to slow at least one time constant describing the intra-activation shut-time histogram and modestly reduce channel open time, which we interpret to reflect a reduction in the overall channel activation rate and possible proton-induced termination of openings. This is consistent with a modest proton-dependent slowing of the macroscopic response rise time. From these data, we propose a physical model of proton inhibition that can describe macroscopic and single-channel properties of NMDA receptor function over a range of pH values.
Figures





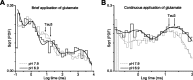

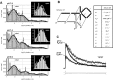
References
-
- Agmon N (1995) The Grotthus mechanism. Chem Phys Lett 244: 456-462.
-
- Banke TG, Traynelis SF (2003) Activation of NR1/NR2B NMDA receptors. Nat Neurosci 6: 144-152. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous
