The influence of pioneer neurons on a growing motor nerve in Drosophila requires the neural cell adhesion molecule homolog FasciclinII
- PMID: 15634769
- PMCID: PMC6725196
- DOI: 10.1523/JNEUROSCI.2377-04.2005
The influence of pioneer neurons on a growing motor nerve in Drosophila requires the neural cell adhesion molecule homolog FasciclinII
Abstract
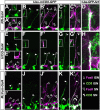
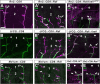
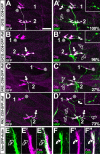
The phenomenon of pioneer neurons has been known for almost a century, but so far we have little insights into mechanisms and molecules involved. Here, we study the formation of the Drosophila intersegmental motor nerve (ISN). We show that aCC/RP2 and U motor neurons grow together at the leading front of the ISN. Nevertheless, aCC/RP2 neurons are the pioneers, and U neurons are the followers, because only aCC/RP2 neurons effectively influence growth of the ISN. We also show that this influence depends on the neural cell adhesion molecule homolog FasciclinII. First, ablation of aCC/RP2 has a stronger impact on ISN growth than U ablation. Second, strong growth-influencing capabilities of aCC/RP2 are revealed with a stalling approach we used: when aCC/RP2 motor axons are stalled specifically, the entire ISN (including the U neurons) coarrests, demonstrating that aCC/RP2 neurons influence the behavior of U growth cones. In contrast, stalled U neurons do not have the same influence on other ISN motor neurons. The influence on ISN growth requires FasciclinII: targeted expression of FasciclinII in U neurons increases their influence on the ISN, whereas a FasciclinII loss-of-function background reduces ISN coarrest with stalled aCC/RP2 axons. The qualitative differences of both neuron groups are confirmed through our findings that aCC/RP2 growth cones are wider and more complex than those of U neurons. However, U growth cones adopt aCC/RP2-like wider shapes in a FasciclinII loss-of-function background. Therefore, FasciclinII is to a degree required and sufficient for pioneer-follower interactions, but its mode of action cannot be explained merely through an equally bidirectional adhesive interaction.
Figures



References
-
- Bak M, Fraser SE (2003) Axon fasciculation and differences in midline kinetics between pioneer and follower axons within commissural fascicles. Development 130: 4999-5008. - PubMed
-
- Bate M (1993) The mesoderm and its derivatives. In: The development of Drosophila melanogaster (Bate M, Martínez Arias A, eds), pp 1013-1090. Cold Spring Harbor, NY: Cold Spring Harbor Laboratory.
-
- Boschert U, Ramos RG, Tix S, Technau GM, Fischbach KF (1990) Genetic and developmental analysis of irreC, a genetic function required for optic chiasm formation in Drosophila. J Neurogenet 6: 153-171. - PubMed
-
- Bowie JE, Young IT (1977) An analysis technique for biological shape-II. Acta Cytol 21: 455-464. - PubMed
-
- Brand A, Perrimon N (1993) Targeted gene expression as a means of altering cell fates and generating dominant phenotypes. Development 118: 401-415. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
