Congenital stationary night blindness type 2 mutations S229P, G369D, L1068P, and W1440X alter channel gating or functional expression of Ca(v)1.4 L-type Ca2+ channels
- PMID: 15634789
- PMCID: PMC6725195
- DOI: 10.1523/JNEUROSCI.3054-04.2005
Congenital stationary night blindness type 2 mutations S229P, G369D, L1068P, and W1440X alter channel gating or functional expression of Ca(v)1.4 L-type Ca2+ channels
Abstract
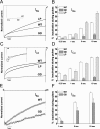
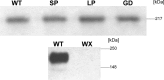
Mutations in the CACNA1F gene (voltage-dependent L-type calcium channel alpha1F subunit) encoding retinal Ca(v)1.4 L-type Ca2+ channels cause X-linked recessive congenital stationary night blindness type 2 (CSNB2). Many of them are predicted to yield nonfunctional channels. Complete loss of Ca(v)1.4 function is therefore regarded as a pathogenetic mechanism for the impaired signaling from photoreceptors to second-order retinal neurons. We investigated the functional consequences of CSNB2 missense mutations S229P, G369D, and L1068P and the C-terminal truncation mutant W1440X. After expression in Xenopus laevis oocytes or human embryonic kidney tsA-201 cells, inward Ca2+ current (I(Ca)) and inward Ba2+ current (I(Ba)) could be recorded from mutations G369D and L1068P. G369D shifted the half-maximal voltage for channel activation (V(0.5,act)) significantly to more negative potentials (>11 mV), slowed inactivation, and removed Ca2+-dependent inactivation. The L1068P mutant yielded currents only in the presence of the channel activator BayK8644. Currents (I(Ba)) inactivated faster than wild type (WT) and recovered more slowly from inactivation (I(Ba) and I(Ca)). No channel activity could be measured for mutants S229P and W1440X after oocyte expression. No W1440X alpha1 protein was detected after expression in tsA-201 cells, whereas S229P (as well as G369D and L1068P) alpha1 subunits were expressed at levels indistinguishable from WT (n = 3). Our data provide unequivocal evidence that CSNB2 missense mutations can induce severe changes in Ca(v)1.4 function, which may decrease (L1068P and S229P) or even increase (G369D) channel activity. The lower activation range of G369D can explain the reduced dynamic range of photoreceptor signaling. Moreover, we demonstrate that loss of channel function of one (L1068P) CSNB2 mutation can be rescued by a Ca2+ channel activator.
Figures



References
-
- Ball SL, Powers PA, Shin HS, Morgans CW, Peachey NS, Gregg RG (2002) Role of the β2 subunit of voltage-dependent calcium channels in the retinal outer plexiform layer. Invest Ophthalmol Vis Sci 43: 1595-1603. - PubMed
-
- Barnes S, Kelly ME (2002) Calcium channels at the photoreceptor synapse. Adv Exp Med Biol 514: 465-476. - PubMed
-
- Baumann L, Gerstner A, Zong X, Biel M, Wahl-Schott C (2004) Functional characterization of the L-type Ca2+ channel Cav1.4 α1 from mouse retina. Invest Ophthalmol Vis Sci 45: 708-713. - PubMed
-
- Bech-Hansen NT, Naylor MJ, Maybaum TA, Pearce WG, Koop B, Fishman GA, Mets M, Musarella MA, Boycott KM (1998) Loss-of-function mutations in a calcium-channel α1-subunit gene in Xp11.23 cause incomplete X-linked congenital stationary night blindness. Nat Genet 19: 264-267. - PubMed
-
- Boycott KM, Pearce WG, Bech-Hansen NT (2000) Clinical variability among patients with incomplete X-linked congenital stationary night blindness and a founder mutation in CACNA1F. Can J Ophthalmol 35: 204-213. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Research Materials
Miscellaneous
