Microbial DNA typing by automated repetitive-sequence-based PCR
- PMID: 15634972
- PMCID: PMC540112
- DOI: 10.1128/JCM.43.1.199-207.2005
Microbial DNA typing by automated repetitive-sequence-based PCR
Abstract
Repetitive sequence-based PCR (rep-PCR) has been recognized as an effective method for bacterial strain typing. Recently, rep-PCR has been commercially adapted to an automated format known as the DiversiLab system to provide a reliable PCR-based typing system for clinical laboratories. We describe the adaptations made to automate rep-PCR and explore the performance and reproducibility of the system as a molecular genotyping tool for bacterial strain typing. The modifications for automation included changes in rep-PCR chemistry and thermal cycling parameters, incorporation of microfluidics-based DNA amplicon fractionation and detection, and Internet-based computer-assisted analysis, reporting, and data storage. The performance and reproducibility of the automated rep-PCR were examined by performing DNA typing and replicate testing with multiple laboratories, personnel, instruments, DNA template concentrations, and culture conditions prior to DNA isolation. Finally, we demonstrated the use of automated rep-PCR for clinical laboratory applications by using isolates from an outbreak of Neisseria meningitidis infections. N. meningitidis outbreak-related strains were distinguished from other isolates. The DiversiLab system is a highly integrated, convenient, and rapid testing platform that may allow clinical laboratories to realize the potential of microbial DNA typing.
Figures
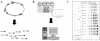






References
-
- Borgdorff, M. W., A. Kolk, D. van Soolingen, J. W. van der Meer, and T. H. Ottenhoff. 2003. Research into new methods for diagnosing, treating and preventing tuberculosis. Ned. Tijdschr. Geneeskd. 147:1838-1841. - PubMed
-
- Cangelosi, G. A., R. J. Freeman, K. N. Lewis, D. Livingston-Rosanoff, K. S. Shah, S. J. Milan, and S. V. Goldberg. 2004. Evaluation of a high-throughput repetitive-sequence-based PCR system for DNA fingerprinting of Mycobacterium tuberculosis and Mycobacterium avium complex strains. J. Clin. Microbiol. 42:2685-2693. - PMC - PubMed
-
- Clementino, M. M., I. de Filippis, C. R. Nascimento, R. Branquinho, C. L. Rocha, and O. B. Martins. 2001. PCR analyses of tRNA intergenic spacer, 16S-23S internal transcribed spacer, and randomly amplified polymorphic DNA reveal inter- and intraspecific relationships of Enterobacter cloacae strains. J. Clin. Microbiol. 39:3865-3870. - PMC - PubMed
-
- Cousins, D. V., R. A. Skuce, R. R. Kazwala, J. D. van Embden, et al. 1998. Towards a standardized approach to DNA fingerprinting of Mycobacterium bovis. Int. J. Tuberc. Lung Dis. 2:471-478. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

