Real-time nucleic acid sequence-based amplification is more convenient than real-time PCR for quantification of Plasmodium falciparum
- PMID: 15635001
- PMCID: PMC540116
- DOI: 10.1128/JCM.43.1.402-405.2005
Real-time nucleic acid sequence-based amplification is more convenient than real-time PCR for quantification of Plasmodium falciparum
Abstract
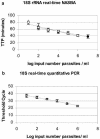
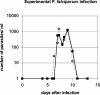
Determination of the number of malaria parasites by routine or even expert microscopy is not always sufficiently sensitive for detailed quantitative studies on the population dynamics of Plasmodium falciparum, such as intervention or vaccine trials. To circumvent this problem, two more sensitive assays, real-time quantitative nucleic acid sequence-based amplification (QT-NASBA) and real-time quantitative PCR (QT-PCR) were compared for quantification of P. falciparum parasites. QT-NASBA was adapted to molecular beacon real-time detection technology, which enables a reduction of the time of analysis and of contamination risk while retaining the specificity and sensitivity of the original assay. Both QT-NASBA and QT-PCR have a sensitivity of 20 parasites/ml of blood, but QT-PCR requires a complicated DNA extraction procedure and the use of 500 microl of venous blood to achieve this sensitivity, compared to 50 microl of finger prick blood for real-time QT-NASBA. Both techniques show a significant correlation to microscopic parasite counts, and the quantification results of the two real-time assays are significantly correlated for in vitro as well as in vivo samples. However, in comparison to real-time QT-PCR, the results of real-time QT-NASBA can be obtained 12 h earlier, with relatively easy RNA extraction and use of finger prick blood samples. The prospective development of multiplex QT-NASBA for detection of various P. falciparum developmental stages increases the value of QT-NASBA for malaria studies. Therefore, for studies requiring sensitive and accurate detection of P. falciparum parasites in large numbers of samples, the use of real-time QT-NASBA is preferred over that of real-time QT-PCR.
Figures
References
-
- Bell, A. S., and L. C. Ranford Cartwright. 2002. Real-time quantitative PCR in parasitology. Trends Parasitol. 18:337-342. - PubMed
-
- Hermsen, C. C., D. S. Telgt, E. H. Linders, L. A. van de Locht, W. M. Eling, E. J. Mensink, and R. W. Sauerwein. 2001. Detection of Plasmodium falciparum malaria parasites in vivo by real-time quantitative PCR. Mol. Biochem. Parasitol. 118:247-251. - PubMed
-
- Ponnudurai, T., A. H. Lensen, J. F. Meis, and J. H. Meuwissen. 1986. Synchronization of Plasmodium falciparum gametocytes using an automated suspension culture system. Parasitology 93:263-274. - PubMed
-
- Schallig, H. D., G. J. Schoone, E. J. Lommerse, C. C. Kroon, P. J. de Vries, and T. van Gool. 2003. Usefulness of quantitative nucleic acid sequence-based amplification for diagnosis of malaria in an academic hospital setting. Eur. J. Clin. Microbiol. Infect. Dis. 22:555-557. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

